The Ghrelin/Growth Hormone Secretagogue Receptor System Is Involved in the Rapid and Sustained Antidepressant-Like Effect of Paeoniflorin
- PMID: 33664648
- PMCID: PMC7920966
- DOI: 10.3389/fnins.2021.631424
The Ghrelin/Growth Hormone Secretagogue Receptor System Is Involved in the Rapid and Sustained Antidepressant-Like Effect of Paeoniflorin
Abstract
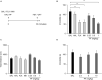
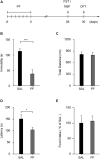
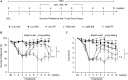
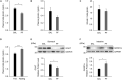
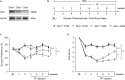
Major depressive disorder (MDD) is a debilitating mental illness affecting people worldwide. Although significant progress has been made in the development of therapeutic agents to treat this condition, fewer than half of all patients respond to currently available antidepressants, highlighting the urgent need for the development of new classes of antidepressant drugs. Here, we found that paeoniflorin (PF) produced rapid and sustained antidepressant-like effects in multiple mouse models of depression, including the forced swimming test and exposure to chronic mild stress (CMS). Moreover, PF decreased the bodyweight of mice without affecting food intake and glucose homeostasis, and also reduced the plasma levels of total ghrelin and the expression of ghrelin O-acyltransferase in the stomach; however, the plasma levels of ghrelin and the ghrelin/total ghrelin ratio were unaffected. Furthermore, PF significantly increased the expression of growth hormone secretagogue receptor 1 alpha (GHSR1α, encoded by the Ghsr gene) in the intestine, whereas the levels of GHSR1α in the brain were only marginally downregulated following subchronic PF treatment. Finally, the genetic deletion of Ghsr attenuated the antidepressant-like effects of PF in mice exposed to CMS. These results suggested that increased GHSR1α expression in the intestine mediates the antidepressant-like effects of PF. Understanding peripheral ghrelin/GHSR signaling may provide new insights for the screening of antidepressant drugs that produce fast-acting and sustained effects.
Keywords: antidepressant; growth hormone secretagogue receptor 1α; intestine; major depressive disorder; paeoniflorin.
Copyright © 2021 Zhang, Zhu, Qin, Zeng and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

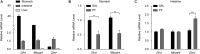

Similar articles
-
Ghrelin/GHSR System in Depressive Disorder: Pathologic Roles and Therapeutic Implications.Curr Issues Mol Biol. 2024 Jul 10;46(7):7324-7338. doi: 10.3390/cimb46070434. Curr Issues Mol Biol. 2024. PMID: 39057075 Free PMC article. Review.
-
Ghrelin/GHSR signaling in the lateral septum ameliorates chronic stress-induced depressive-like behaviors.Prog Neuropsychopharmacol Biol Psychiatry. 2024 Apr 20;131:110953. doi: 10.1016/j.pnpbp.2024.110953. Epub 2024 Jan 24. Prog Neuropsychopharmacol Biol Psychiatry. 2024. PMID: 38278286
-
Ghrelin alleviates anxiety- and depression-like behaviors induced by chronic unpredictable mild stress in rodents.Behav Brain Res. 2017 May 30;326:33-43. doi: 10.1016/j.bbr.2017.02.040. Epub 2017 Feb 27. Behav Brain Res. 2017. PMID: 28245976
-
Rapid and Prolonged Antidepressant-like Effect of Crocin Is Associated with GHSR-Mediated Hippocampal Plasticity-related Proteins in Mice Exposed to Prenatal Stress.ACS Chem Neurosci. 2020 Apr 15;11(8):1159-1170. doi: 10.1021/acschemneuro.0c00022. Epub 2020 Mar 31. ACS Chem Neurosci. 2020. PMID: 32203651
-
Ghrelin and the growth hormone secretagogue receptor in growth and development.Int J Obes (Lond). 2009 Apr;33 Suppl 1:S48-52. doi: 10.1038/ijo.2009.17. Int J Obes (Lond). 2009. PMID: 19363508 Review.
Cited by
-
A review for the pharmacological effects of paeoniflorin in the nervous system.Front Pharmacol. 2022 Aug 15;13:898955. doi: 10.3389/fphar.2022.898955. eCollection 2022. Front Pharmacol. 2022. PMID: 36046834 Free PMC article. Review.
-
Ghrelin/GHSR System in Depressive Disorder: Pathologic Roles and Therapeutic Implications.Curr Issues Mol Biol. 2024 Jul 10;46(7):7324-7338. doi: 10.3390/cimb46070434. Curr Issues Mol Biol. 2024. PMID: 39057075 Free PMC article. Review.
-
Total Triterpenes of Wolfiporia cocos (Schwein.) Ryvarden & Gilb Exerts Antidepressant-Like Effects in a Chronic Unpredictable Mild Stress Rat Model and Regulates the Levels of Neurotransmitters, HPA Axis and NLRP3 Pathway.Front Pharmacol. 2022 Feb 14;13:793525. doi: 10.3389/fphar.2022.793525. eCollection 2022. Front Pharmacol. 2022. PMID: 35237160 Free PMC article.
-
Ghrelin, Neuroinflammation, Oxidative Stress, and Mood Disorders: What Are the Connections?Curr Neuropharmacol. 2025;23(2):172-186. doi: 10.2174/1570159X22999240722095039. Curr Neuropharmacol. 2025. PMID: 39041263 Free PMC article. Review.
-
Growth hormone and IGF-1 actions in the brain and neuropsychiatric diseases.Physiology (Bethesda). 2025 Jul 7:10.1152/physiol.00009.2025. doi: 10.1152/physiol.00009.2025. Online ahead of print. Physiology (Bethesda). 2025. PMID: 40623083 Free PMC article. Review.
References
-
- Agawa S., Futagami S., Yamawaki H., Ikeda G., Noda H., Kirita K., et al. (2019). Acylated ghrelin levels were associated with depressive status, physical quality of life, endoscopic findings based on Kyoto classification in Japan. J. Clin. Biochem. Nutr. 65 65–70. 10.3164/jcbn.18-111 - DOI - PMC - PubMed
-
- Burokas A., Arboleya S., Moloney R. D., Peterson V. L., Murphy K., Clarke G., et al. (2017). Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 82 472–487. 10.1016/j.biopsych.2016.12.031 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

