Roles for the Dorsal Striatum in Aversive Behavior
- PMID: 33664651
- PMCID: PMC7920955
- DOI: 10.3389/fncel.2021.634493
Roles for the Dorsal Striatum in Aversive Behavior
Abstract
The ability to identify and avoid environmental stimuli that signal danger is essential to survival. Our understanding of how the brain encodes aversive behaviors has been primarily focused on roles for the amygdala, hippocampus (HIPP), prefrontal cortex, ventral midbrain, and ventral striatum. Relatively little attention has been paid to contributions from the dorsal striatum (DS) to aversive learning, despite its well-established role in stimulus-response learning. Here, we review studies exploring the role of DS in aversive learning, including different roles for the dorsomedial and dorsolateral striatum in Pavlovian fear conditioning as well as innate and inhibitory avoidance (IA) behaviors. We outline how future investigation might determine specific contributions from DS subregions, cell types, and connections that contribute to aversive behavior.
Keywords: aversion; dorsal striatum; fear conditioning; inhibitory avoidance; threat.
Copyright © 2021 Stanley, Lippiello, Sulzer and Miniaci.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
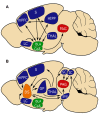
Figures

Similar articles
-
Dopaminergic circuits underlying associative aversive learning.Front Behav Neurosci. 2022 Nov 10;16:1041929. doi: 10.3389/fnbeh.2022.1041929. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36439963 Free PMC article. Review.
-
Dissociable Learning Processes Underlie Human Pain Conditioning.Curr Biol. 2016 Jan 11;26(1):52-8. doi: 10.1016/j.cub.2015.10.066. Epub 2015 Dec 17. Curr Biol. 2016. PMID: 26711494 Free PMC article.
-
Activation of the ventral striatum during aversive contextual conditioning in humans.Biol Psychol. 2012 Sep;91(1):74-80. doi: 10.1016/j.biopsycho.2012.04.004. Epub 2012 Apr 26. Biol Psychol. 2012. PMID: 22560888
-
The consolidation of inhibitory avoidance memory in mice depends on the intensity of the aversive stimulus: The involvement of the amygdala, dorsal hippocampus and medial prefrontal cortex.Neurobiol Learn Mem. 2016 Apr;130:44-51. doi: 10.1016/j.nlm.2016.01.012. Epub 2016 Feb 2. Neurobiol Learn Mem. 2016. PMID: 26851130
-
Diverse Effects of Conditioned Threat Stimuli on Behavior.Cold Spring Harb Symp Quant Biol. 2014;79:11-9. doi: 10.1101/sqb.2014.79.024968. Epub 2015 Feb 19. Cold Spring Harb Symp Quant Biol. 2014. PMID: 25699986 Review.
Cited by
-
The Relationship Between Brain Activation for Taking Others' Perspective and Interoceptive Abilities in Autism Spectrum Disorder: An fMRI Study.J Korean Acad Child Adolesc Psychiatry. 2024 Jul 1;35(3):197-209. doi: 10.5765/jkacap.240008. J Korean Acad Child Adolesc Psychiatry. 2024. PMID: 38966201 Free PMC article.
-
High ovarian hormones present during fear extinction reduce fear relapse through a nigrostriatal dopamine pathway.Biol Sex Differ. 2025 Jun 1;16(1):38. doi: 10.1186/s13293-025-00722-7. Biol Sex Differ. 2025. PMID: 40452046 Free PMC article.
-
Dopaminergic circuits underlying associative aversive learning.Front Behav Neurosci. 2022 Nov 10;16:1041929. doi: 10.3389/fnbeh.2022.1041929. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36439963 Free PMC article. Review.
-
Altered anticipatory brain responses in eating disorders: A neuroimaging meta-analysis.Eur Eat Disord Rev. 2023 May;31(3):363-376. doi: 10.1002/erv.2967. Epub 2023 Jan 13. Eur Eat Disord Rev. 2023. PMID: 36639902 Free PMC article. Review.
-
Pharmacological manipulations of the dorsomedial and dorsolateral striatum during fear extinction reveal opposing roles in fear renewal.Neurobiol Learn Mem. 2024 Jul;212:107937. doi: 10.1016/j.nlm.2024.107937. Epub 2024 May 11. Neurobiol Learn Mem. 2024. PMID: 38735637 Free PMC article.
References
-
- Almada R. C., Genewsky A. J., Heinz D. E., Kaplick P. M., Coimbra N. C., Wotjak C. T. (2018). Stimulation of the nigrotectal pathway at the level of the superior colliculus reduces threat recognition and causes a shift from avoidance to approach behavior. Front. Neural Circuits 12:36. 10.3389/fncir.2018.00036 - DOI - PMC - PubMed
-
- Bello-Medina P. C., Flores G., Quirarte G. L., McGaugh J. L., Prado Alcalá R. A. (2016). Mushroom spine dynamics in medium spiny neurons of dorsal striatum associated with memory of moderate and intense training. Proc. Natl. Acad. Sci. U S A 113, E6516–E6525. 10.1073/pnas.1613680113 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

