A Novel Mechanism Regulating Dopamine Receptor Type 2 Signal Transduction in Pituitary Tumoral Cells: The Role of cAMP/PKA-Induced Filamin A Phosphorylation
- PMID: 33664708
- PMCID: PMC7921166
- DOI: 10.3389/fendo.2020.611752
A Novel Mechanism Regulating Dopamine Receptor Type 2 Signal Transduction in Pituitary Tumoral Cells: The Role of cAMP/PKA-Induced Filamin A Phosphorylation
Abstract
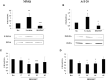
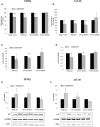
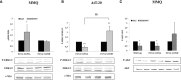
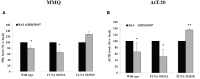
The actin binding protein filamin A (FLNA) is required for somatostatin receptor 2 (SSTR2) and dopamine receptor 2 (DRD2) expression and signaling in GH- and PRL-secreting PitNETs, respectively, playing a role in tumor responsiveness to somatostatin receptors ligands and dopaminergic drugs. FLNA functions are regulated by several mechanisms, including phosphorylation. It has been shown that in GH-secreting PitNETs FLNA phosphorylation on Ser2152 (P-FLNA) switches FLNA function from a scaffold that allows SSTR2 signal transduction, to a signal termination protein that hampers SSTR2 antitumoral effects. Aims of the present study were to evaluate in PRL- and ACTH-secreting PitNETs cell lines MMQ and AtT-20 the effects of cAMP pathway activation and DRD2 agonist on P-FLNA and the impact of P-FLNA on DRD2 signal transduction. We found that forskolin increased (+2.2 ± 0.8-fold, p < 0.01 in MMQ; +1.9 ± 0.58-fold, p < 0.05 in AtT-20), and DRD2 agonist BIM53097 reduced (-49.4 ± 25%, p < 0.001 in MMQ; -45.8 ± 28%, p < 0.05 in AtT-20), P-FLNA on Ser2152. The overexpression of a phosphomimetic (S2152D) FLNA mutant in both cell lines prevented DRD2 antiproliferative effects, that were comparable in cells transfected with empty vector, wild-type FLNA as well as phosphodeficient FLNA mutant (S2152A) (-20.6 ± 5% cell proliferation, p < 0.001 in MMQ; -36.6 ± 12%, p < 0.01 in AtT-20). Accordingly, S2152D FLNA expression abolished the expected ability of BIM53097 to increase or decrease, in MMQ and in AtT20 respectively, ERK phosphorylation, an effect that was maintained in S2152A FLNA expressing cells (+1.8 ± 0.65-fold, p < 0.05 in MMQ; -55 ± 13%, p < 0.01 in AtT-20). In addition, the inhibitory effects of DRD2 on hormone secretion (-34.3 ± 6% PRL, p < 0.05 in MMQ; -42.8 ± 22% ACTH, p < 0.05 in AtT-20, in cells expressing S2152A FLNA) were completely lost in S2152D FLNA transfected cells. In conclusion, our data demonstrated that cAMP pathway and DRD2 agonist regulated FLNA activity by increasing or decreasing, respectively, its phosphorylation. Moreover, we found that P-FLNA prevented DRD2 signaling in PRL- and ACTH-secreting tumoral pituitary cell lines, suggesting that this FLNA modification might represent a new regulatory mechanism shared by different GPCRs. In PitNETs expressing DRD2, modulation of P-FLNA might suggest new pharmacological strategies to overcome drug resistance, and P-FLNA might represent a new biomarker for tumor responsiveness to dopaminergic agents.
Keywords: cAMP/PKA pathway; dopamine receptor type 2; filamin A phosphorylation; pituitary neuroendocrine tumors; signal transduction.
Copyright © 2021 Mangili, Treppiedi, Catalano, Marra, Di Muro, Spada, Arosio, Peverelli and Mantovani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
cAMP/PKA-induced filamin A (FLNA) phosphorylation inhibits SST2 signal transduction in GH-secreting pituitary tumor cells.Cancer Lett. 2018 Oct 28;435:101-109. doi: 10.1016/j.canlet.2018.08.002. Epub 2018 Aug 8. Cancer Lett. 2018. PMID: 30098401
-
Beta-Arrestin 2 Is Required for Dopamine Receptor Type 2 Inhibitory Effects on AKT Phosphorylation and Cell Proliferation in Pituitary Tumors.Neuroendocrinology. 2021;111(6):568-579. doi: 10.1159/000509219. Epub 2020 Jun 8. Neuroendocrinology. 2021. PMID: 32512568
-
Filamin A in somatostatin and dopamine receptor regulation in pituitary and the role of cAMP/PKA dependent phosphorylation.Horm Metab Res. 2014 Nov;46(12):845-53. doi: 10.1055/s-0034-1384520. Epub 2014 Jul 28. Horm Metab Res. 2014. PMID: 25068602 Review.
-
A novel pathway activated by somatostatin receptor type 2 (SST2): Inhibition of pituitary tumor cell migration and invasion through cytoskeleton protein recruitment.Int J Cancer. 2018 May 1;142(9):1842-1852. doi: 10.1002/ijc.31205. Epub 2017 Dec 20. Int J Cancer. 2018. PMID: 29226331
-
Cytoskeleton actin-binding proteins in clinical behavior of pituitary tumors.Endocr Relat Cancer. 2019 Feb 1;26(2):R95-R108. doi: 10.1530/ERC-18-0442. Endocr Relat Cancer. 2019. PMID: 30589642 Review.
Cited by
-
E prostanoid receptor-3 promotes oxidized low-density lipoprotein-induced human aortic smooth muscle cells inflammation.ESC Heart Fail. 2023 Apr;10(2):1077-1089. doi: 10.1002/ehf2.14264. Epub 2022 Dec 28. ESC Heart Fail. 2023. PMID: 36578105 Free PMC article.
-
Charting the importance of filamin A posttranslational modifications.Biochem J. 2024 Jul 3;481(13):865-881. doi: 10.1042/BCJ20240121. Biochem J. 2024. PMID: 38958472 Free PMC article. Review.
-
Molecular characterization of epithelial-mesenchymal transition and medical treatment related-genes in non-functioning pituitary neuroendocrine tumors.Front Endocrinol (Lausanne). 2023 Mar 22;14:1129213. doi: 10.3389/fendo.2023.1129213. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37033229 Free PMC article.
-
Immunohistochemical evaluation of biomarkers with predictive role in acromegaly: a literature review.Rom J Morphol Embryol. 2023 Jan-Mar;64(1):25-33. doi: 10.47162/RJME.64.1.03. Rom J Morphol Embryol. 2023. PMID: 37128788 Free PMC article. Review.
-
Molecular mechanisms involved in somatostatin receptor regulation in corticotroph tumors: the role of cytoskeleton and USP8 mutations.Endocr Oncol. 2022 Mar 23;2(1):R24-R30. doi: 10.1530/EO-22-0042. eCollection 2022 Jan. Endocr Oncol. 2022. PMID: 37435448 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

