Poly(dA:dT) Suppresses HSV-2 Infection of Human Cervical Epithelial Cells Through RIG-I Activation
- PMID: 33664729
- PMCID: PMC7923882
- DOI: 10.3389/fimmu.2020.598884
Poly(dA:dT) Suppresses HSV-2 Infection of Human Cervical Epithelial Cells Through RIG-I Activation
Abstract
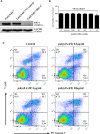
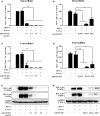
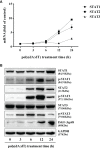
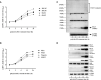
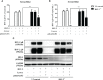
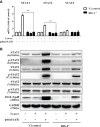
Epithelial cells of the female reproductive tract (FRT) participate in the initial innate immunity against viral infections. Poly(dA:dT) is a synthetic analog of B form double-stranded (ds) DNA which can activate the interferon (IFN) signaling pathway-mediated antiviral immunity through DNA-dependent RNA Polymerase III. Here we investigated whether poly(dA:dT) could inhibit herpes simplex virus type 2 (HSV-2) infection of human cervical epithelial cells (End1/E6E7). We demonstrated that poly(dA:dT) treatment of End1/E6E7 cells could significantly inhibit HSV-2 infection. Mechanistically, poly(dA:dT) treatment of the cells induced the expression of the intracellular IFNs and the multiple antiviral IFN-stimulated genes (ISGs), including IFN-stimulated gene 15 (ISG15), IFN-stimulated gene 56 (ISG56), 2'-5'-oligoadenylate synthetase 1 (OAS1), 2'-5'-oligoadenylate synthetase 2 (OAS2), myxovirus resistance protein A (MxA), myxovirus resistance protein B (MxB), virus inhibitory protein, endoplasmic reticulum-associated, IFN-inducible (Viperin), and guanylate binding protein 5 (GBP5). Further investigation showed that the activation of RIG-I was largely responsible for poly(dA:dT)-mediated HSV-2 inhibition and IFN/ISGs induction in the cervical epithelial cells, as RIG-I knockout abolished the poly(dA:dT) actions. These observations demonstrate the importance for design and development of AT-rich dsDNA-based intervention strategies to control HSV-2 mucosal transmission in FRT.
Keywords: herpes simplex virus type 2; human cervical epithelial cells; interferon; interferon-stimulated gene; poly(dA:dT); retinoic acid-inducible gene-I.
Copyright © 2021 Shao, Meng, Liu, Xu, Wang, Hu, Hou and Ho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Cytosolic DNA sensors activation of human astrocytes inhibits herpes simplex virus through IRF1 induction.Front Cell Infect Microbiol. 2024 May 14;14:1383811. doi: 10.3389/fcimb.2024.1383811. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38808062 Free PMC article.
-
Induction of interferon-λ contributes to TLR3 and RIG-I activation-mediated inhibition of herpes simplex virus type 2 replication in human cervical epithelial cells.Mol Hum Reprod. 2015 Dec;21(12):917-29. doi: 10.1093/molehr/gav058. Epub 2015 Oct 26. Mol Hum Reprod. 2015. PMID: 26502803 Free PMC article.
-
IL-22 suppresses HSV-2 replication in human cervical epithelial cells.Cytokine. 2019 Nov;123:154776. doi: 10.1016/j.cyto.2019.154776. Epub 2019 Jul 22. Cytokine. 2019. PMID: 31344598 Free PMC article.
-
Immunoregulatory Functions of Interferons During Genital HSV-2 Infection.Front Immunol. 2021 Aug 18;12:724618. doi: 10.3389/fimmu.2021.724618. eCollection 2021. Front Immunol. 2021. PMID: 34484233 Free PMC article. Review.
-
The Molecular Basis of Viral Inhibition of IRF- and STAT-Dependent Immune Responses.Front Immunol. 2019 Jan 8;9:3086. doi: 10.3389/fimmu.2018.03086. eCollection 2018. Front Immunol. 2019. PMID: 30671058 Free PMC article. Review.
Cited by
-
Cytosolic DNA sensors activation of human astrocytes inhibits herpes simplex virus through IRF1 induction.Front Cell Infect Microbiol. 2024 May 14;14:1383811. doi: 10.3389/fcimb.2024.1383811. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38808062 Free PMC article.
-
The miR-29-3p family suppresses inflammatory osteolysis.J Cell Physiol. 2024 Aug;239(8):e31299. doi: 10.1002/jcp.31299. Epub 2024 May 19. J Cell Physiol. 2024. PMID: 38764231 Free PMC article.
-
TIME Is Ticking for Cervical Cancer.Biology (Basel). 2023 Jun 30;12(7):941. doi: 10.3390/biology12070941. Biology (Basel). 2023. PMID: 37508372 Free PMC article. Review.
-
A Systematic Review of Second-Line Treatments in Antiviral Resistant Strains of HSV-1, HSV-2, and VZV.Cureus. 2023 Mar 9;15(3):e35958. doi: 10.7759/cureus.35958. eCollection 2023 Mar. Cureus. 2023. PMID: 37041924 Free PMC article. Review.
-
CCL28 Enhances HSV-2 gB-Specific Th1-Polarized Immune Responses against Lethal Vaginal Challenge in Mice.Vaccines (Basel). 2022 Aug 10;10(8):1291. doi: 10.3390/vaccines10081291. Vaccines (Basel). 2022. PMID: 36016177 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

