CCR2 Is Dispensable for Disease Resolution but Required for the Restoration of Leukocyte Homeostasis Upon Experimental Malaria-Associated Acute Respiratory Distress Syndrome
- PMID: 33664739
- PMCID: PMC7921736
- DOI: 10.3389/fimmu.2020.628643
CCR2 Is Dispensable for Disease Resolution but Required for the Restoration of Leukocyte Homeostasis Upon Experimental Malaria-Associated Acute Respiratory Distress Syndrome
Erratum in
-
Corrigendum: CCR2 Is Dispensable for Disease Resolution but Required for the Restoration of Leukocyte Homeostasis Upon Experimental Malaria-Associated Acute Respiratory Distress Syndrome.Front Immunol. 2022 Jun 1;13:926032. doi: 10.3389/fimmu.2022.926032. eCollection 2022. Front Immunol. 2022. PMID: 35720305 Free PMC article.
Abstract
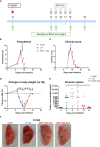
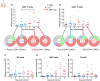
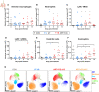
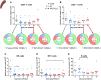
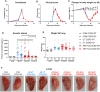
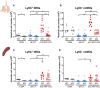
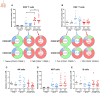
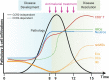
Malaria complications are often lethal, despite efficient killing of Plasmodium parasites with antimalarial drugs. This indicates the need to study the resolution and healing mechanisms involved in the recovery from these complications. Plasmodium berghei NK65-infected C57BL/6 mice develop malaria-associated acute respiratory distress syndrome (MA-ARDS) at 8 days post infection. Antimalarial treatment was started on this day and resulted in the recovery, as measured by the disappearance of the signs of pathology, in >80% of the mice. Therefore, this optimized model represents an asset in the study of mechanisms and leukocyte populations involved in the resolution of MA-ARDS. C-C chemokine receptor type 2 (CCR2) knock-out mice were used to investigate the role of monocytes and macrophages, since these cells are described to play an important role during the resolution of other inflammatory diseases. CCR2 deficiency was associated with significantly lower numbers of inflammatory monocytes in the lungs during infection and resolution and abolished the increase in non-classical monocytes during resolution. Surprisingly, CCR2 was dispensable for the development and the resolution of MA-ARDS, since no effect of the CCR2 knock-out was observed on any of the disease parameters. In contrast, the reappearance of eosinophils and interstitial macrophages during resolution was mitigated in the lungs of CCR2 knock-out mice. In conclusion, CCR2 is required for re-establishing the homeostasis of pulmonary leukocytes during recovery. Furthermore, the resolution of malaria-induced lung pathology is mediated by unknown CCR2-independent mechanisms.
Keywords: eosinophils; immunology; inflammation; malaria; monocytes; parasitology; resolution.
Copyright © 2021 Pollenus, Pham, Vandermosten, Possemiers, Knoops, Opdenakker and Van den Steen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Experimental Models to Study the Pathogenesis of Malaria-Associated Acute Respiratory Distress Syndrome.Front Cell Infect Microbiol. 2022 May 23;12:899581. doi: 10.3389/fcimb.2022.899581. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35677654 Free PMC article. Review.
-
NK cells contribute to the resolution of experimental malaria-associated acute respiratory distress syndrome after antimalarial treatment.Front Immunol. 2024 Sep 17;15:1433904. doi: 10.3389/fimmu.2024.1433904. eCollection 2024. Front Immunol. 2024. PMID: 39355242 Free PMC article.
-
The emergence of pathogenic TNF/iNOS producing dendritic cells (Tip-DCs) in a malaria model of acute respiratory distress syndrome (ARDS) is dependent on CCR4.Mucosal Immunol. 2019 Mar;12(2):312-322. doi: 10.1038/s41385-018-0093-5. Epub 2018 Oct 18. Mucosal Immunol. 2019. PMID: 30337650 Free PMC article.
-
Experimental malaria-associated acute respiratory distress syndrome is dependent on the parasite-host combination and coincides with normocyte invasion.Malar J. 2018 Mar 5;17(1):102. doi: 10.1186/s12936-018-2251-3. Malar J. 2018. PMID: 29506544 Free PMC article.
-
Integrin αDβ2 (CD11d/CD18) mediates experimental malaria-associated acute respiratory distress syndrome (MA-ARDS).Malar J. 2016 Jul 30;15(1):393. doi: 10.1186/s12936-016-1447-7. Malar J. 2016. PMID: 27473068 Free PMC article.
Cited by
-
Absence of CCR2 Promotes Proliferation of Alveolar Macrophages That Control Lung Inflammation in Acute Respiratory Distress Syndrome in Mice.Int J Mol Sci. 2022 Oct 26;23(21):12920. doi: 10.3390/ijms232112920. Int J Mol Sci. 2022. PMID: 36361722 Free PMC article.
-
Skeleton binding protein-1-mediated parasite sequestration inhibits spontaneous resolution of malaria-associated acute respiratory distress syndrome.PLoS Pathog. 2021 Nov 29;17(11):e1010114. doi: 10.1371/journal.ppat.1010114. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34843584 Free PMC article.
-
JAK/STAT inhibition protects glucocorticoid receptor knockout mice from lethal malaria-induced hypoglycemia and hyperinflammation.EMBO Mol Med. 2025 Aug;17(8):2040-2070. doi: 10.1038/s44321-025-00264-w. Epub 2025 Jul 23. EMBO Mol Med. 2025. PMID: 40702267 Free PMC article.
-
Mouse and human macrophages and their roles in cardiovascular health and disease.Nat Cardiovasc Res. 2024 Dec;3(12):1424-1437. doi: 10.1038/s44161-024-00580-3. Epub 2024 Nov 27. Nat Cardiovasc Res. 2024. PMID: 39604762 Review.
-
Experimental Models to Study the Pathogenesis of Malaria-Associated Acute Respiratory Distress Syndrome.Front Cell Infect Microbiol. 2022 May 23;12:899581. doi: 10.3389/fcimb.2022.899581. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35677654 Free PMC article. Review.
References
-
- World Health Organization . WHO World Malaria Report 2020. World Health Organization. (2020)978 92 4 1564403.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

