Update on the Roles of Polyamines in Fleshy Fruit Ripening, Senescence, and Quality
- PMID: 33664757
- PMCID: PMC7922164
- DOI: 10.3389/fpls.2021.610313
Update on the Roles of Polyamines in Fleshy Fruit Ripening, Senescence, and Quality
Abstract
Ripening of fleshy fruits involves complex physiological, biochemical, and molecular processes that coincide with various changes of the fruit, including texture, color, flavor, and aroma. The processes of ripening are controlled by ethylene in climacteric fruits and abscisic acid (ABA) in non-climacteric fruits. Increasing evidence is also uncovering an essential role for polyamines (PAs) in fruit ripening, especially in climacteric fruits. However, until recently breakthroughs have been made in understanding PA roles in the ripening of non-climacteric fruits. In this review, we compare the mechanisms underlying PA biosynthesis, metabolism, and action during ripening in climacteric and non-climacteric fruits at the physiological and molecular levels. The PA putrescine (Put) has a role opposite to that of spermidine/spermine (Spd/Spm) in cellular metabolism. Arginine decarboxylase (ADC) is crucial to Put biosynthesis in both climacteric and non-climacteric fruits. S-adenosylmethionine decarboxylase (SAMDC) catalyzes the conversion of Put to Spd/Spm, which marks a metabolic transition that is concomitant with the onset of fruit ripening, induced by Spd in climacteric fruits and by Spm in non-climacteric fruits. Once PA catabolism is activated by polyamine oxidase (PAO), fruit ripening and senescence are facilitated by the coordination of mechanisms that involve PAs, hydrogen peroxide (H2O2), ABA, ethylene, nitric oxide (NO), and calcium ions (Ca2+). Notably, a signal derived from PAO5-mediated PA metabolism has recently been identified in strawberry, a model system for non-climacteric fruits, providing a deeper understanding of the regulatory roles played by PAs in fleshy fruit ripening.
Keywords: ABA; Ca2+; NO; ethylene; fruit ripening; polyamines; review.
Copyright © 2021 Gao, Mei, Li, Guo and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
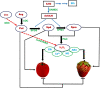
 represent promotion and inhibition, respectively. PA, polyamine; Put, putrescine; Spd, spermidine; Spm, spermine; Arg: arginine; Org, ornithine; SAM, S-adenosyl-L-methionine; dcSAM, decarboxylated SAM; ODC, ornithine decarboxylase; CuAO/DAO, copper amine oxidase/diamine oxidases; PAO, polyamine oxidases; ADC, arginine decarboxylase; SPDS, Spd synthase; SPMS, Spm synthase; SAMDC, SAM decarboxylase; Eth, ethylene; ABA, abscisic acid; NO, nitric oxide; H2O2, hydrogen peroxide.
represent promotion and inhibition, respectively. PA, polyamine; Put, putrescine; Spd, spermidine; Spm, spermine; Arg: arginine; Org, ornithine; SAM, S-adenosyl-L-methionine; dcSAM, decarboxylated SAM; ODC, ornithine decarboxylase; CuAO/DAO, copper amine oxidase/diamine oxidases; PAO, polyamine oxidases; ADC, arginine decarboxylase; SPDS, Spd synthase; SPMS, Spm synthase; SAMDC, SAM decarboxylase; Eth, ethylene; ABA, abscisic acid; NO, nitric oxide; H2O2, hydrogen peroxide.Similar articles
-
Polyamines Regulate Strawberry Fruit Ripening by Abscisic Acid, Auxin, and Ethylene.Plant Physiol. 2018 May;177(1):339-351. doi: 10.1104/pp.18.00245. Epub 2018 Mar 9. Plant Physiol. 2018. PMID: 29523717 Free PMC article.
-
FaPAO5 regulates Spm/Spd levels as a signaling during strawberry fruit ripening.Plant Direct. 2020 Apr 29;4(5):e00217. doi: 10.1002/pld3.217. eCollection 2020 May. Plant Direct. 2020. PMID: 32355906 Free PMC article.
-
The Physiological and Molecular Mechanism of Abscisic Acid in Regulation of Fleshy Fruit Ripening.Front Plant Sci. 2021 Jan 11;11:619953. doi: 10.3389/fpls.2020.619953. eCollection 2020. Front Plant Sci. 2021. PMID: 33505417 Free PMC article. Review.
-
Enriched endogenous free Spd and Spm in alfalfa (Medicago sativa L.) under drought stress enhance drought tolerance by inhibiting H2O2 production to increase antioxidant enzyme activity.J Plant Physiol. 2023 Dec;291:154139. doi: 10.1016/j.jplph.2023.154139. Epub 2023 Nov 16. J Plant Physiol. 2023. PMID: 37988872
-
Non-climacteric fruit development and ripening regulation: 'the phytohormones show'.J Exp Bot. 2023 Oct 31;74(20):6237-6253. doi: 10.1093/jxb/erad271. J Exp Bot. 2023. PMID: 37449770 Free PMC article. Review.
Cited by
-
Genome and transcriptome-wide study of carbamoyltransferase genes in major fleshy fruits: A multi-omics study of evolution and functional significance.Front Plant Sci. 2022 Nov 3;13:994159. doi: 10.3389/fpls.2022.994159. eCollection 2022. Front Plant Sci. 2022. PMID: 36407603 Free PMC article.
-
Whole-Transcriptome Analysis Reveals Autophagy Is Involved in Early Senescence of zj-es Mutant Rice.Front Plant Sci. 2022 Jun 3;13:899054. doi: 10.3389/fpls.2022.899054. eCollection 2022. Front Plant Sci. 2022. PMID: 35720578 Free PMC article.
-
Polyamines: pleiotropic molecules regulating plant development and enhancing crop yield and quality.Plant Biotechnol J. 2024 Nov;22(11):3194-3201. doi: 10.1111/pbi.14440. Epub 2024 Jul 18. Plant Biotechnol J. 2024. PMID: 39024414 Free PMC article. Review.
-
COP9 SIGNALOSOME SUBUNIT 5A facilitates POLYAMINE OXIDASE 5 degradation to regulate strawberry plant growth and fruit ripening.Plant Cell. 2025 Feb 13;37(2):koaf022. doi: 10.1093/plcell/koaf022. Plant Cell. 2025. PMID: 39899466
-
Ripening of Pomegranate Skin as Revealed by Developmental Transcriptomics.Cells. 2022 Jul 16;11(14):2215. doi: 10.3390/cells11142215. Cells. 2022. PMID: 35883658 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

