Identification of Metal Stresses in Arabidopsis thaliana Using Hyperspectral Reflectance Imaging
- PMID: 33664759
- PMCID: PMC7921809
- DOI: 10.3389/fpls.2021.624656
Identification of Metal Stresses in Arabidopsis thaliana Using Hyperspectral Reflectance Imaging
Abstract
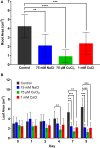
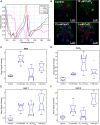
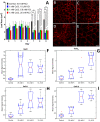
Industrial accidents, such as the Fukushima and Chernobyl disasters, release harmful chemicals into the environment, covering large geographical areas. Natural flora may serve as biological sensors for detecting metal contamination, such as cesium. Spectral detection of plant stresses typically employs a few select wavelengths and often cannot distinguish between different stress phenotypes. In this study, we apply hyperspectral reflectance imaging in the visible and near-infrared along with multivariate curve resolution (MCR) analysis to identify unique spectral signatures of three stresses in Arabidopsis thaliana: salt, copper, and cesium. While all stress conditions result in common stress physiology, hyperspectral reflectance imaging and MCR analysis produced unique spectral signatures that enabled classification of each stress. As the level of potassium was previously shown to affect cesium stress in plants, the response of A. thaliana to cesium stress under variable levels of potassium was also investigated. Increased levels of potassium reduced the spectral response of A. thaliana to cesium and prevented changes to chloroplast cellular organization. While metal stress mechanisms may vary under different environmental conditions, this study demonstrates that hyperspectral reflectance imaging with MCR analysis can distinguish metal stress phenotypes, providing the potential to detect metal contamination across large geographical areas.
Keywords: Arabidopsis; cesium stress; copper stress; hyperspectral imaging; metal stress; multivariate curve resolution; plant hyperspectral imaging; salt stress.
Copyright © 2021 Ruffing, Anthony, Strickland, Lubkin and Dietz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Asaari M. S. M., Mishra P., Mertens S., Dhondt S., Inzé D., Wuyts N., et al. (2018). Close-range hyperspectral image analysis for the early detection of stress responses in individual plants in a high-throughput phenotyping platform. ISPRS J. Photogr. Remote Sens. 138 121–138. 10.1016/j.isprsjprs.2018.02.003 - DOI
-
- Bannari A., Morin D., Bonn F., Huete A. (1995). A review of vegetation indices. Remote Sens. Rev. 13 95–120.
-
- Bertrand M., Schoefs B. (1999). “Photosynthetic pigment metabolism in plants during stress,” in Handbook of Plant and Crop Stress, ed. Pessarakli M. (New York, NY: Marcel Dekker, Inc.), 527–544. 10.1201/9780824746728.ch23 - DOI
-
- Bohnenkamp D., Kuska M., Mahlein A. K., Behmann J. (2019). Hyperspectral signal decomposition and symptom detection of wheat rust disease at the leaf scale using pure fungal spore spectra as reference. Plant Pathol. 68 1188–1195. 10.1111/ppa.13020 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

