Maintenance of variation in virulence and reproduction in populations of an agricultural plant pathogen
- PMID: 33664780
- PMCID: PMC7896723
- DOI: 10.1111/eva.13117
Maintenance of variation in virulence and reproduction in populations of an agricultural plant pathogen
Abstract
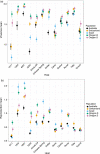
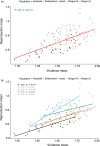
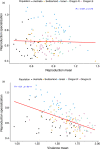
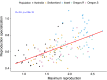
Genetic diversity within pathogen populations is critically important for predicting pathogen evolution, disease outcomes and prevalence. However, we lack a good understanding of the processes maintaining genetic variation and constraints on pathogen life-history evolution. Here, we analysed interactions between 12 wheat host genotypes and 145 strains of Zymoseptoria tritici from five global populations to investigate the evolution and maintenance of variation in pathogen virulence and reproduction. We found a strong positive correlation between virulence (amount of leaf necrosis) and reproduction (pycnidia density within lesions), with substantial variation in both traits maintained within populations. On average, highly virulent isolates exhibited higher reproduction, which might increase transmission potential in agricultural fields planted to homogeneous hosts at a high density. We further showed that pathogen strains with a narrow host range (i.e. specialists) for reproduction were on average less virulent, and those with a broader host range (i.e. generalists) were on average less fecund on a given specific host. These costs associated with adaptation to different host genotypes might constrain the emergence of generalists by disrupting the directional evolution of virulence and fecundity. We conclude that selection favouring pathogen strains that are virulent across diverse hosts, coupled with selection that maximizes fecundity on specific hosts, may explain the maintenance of these pathogenicity traits within and among populations.
Keywords: Zymoseptoria tritici; host specialization; reproduction; trade‐off; virulence; wheat.
© 2020 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Virulence Associations and Global Context of AvrStb6 Genetic Diversity in Iranian Populations of Zymoseptoria tritici.Phytopathology. 2023 Oct;113(10):1924-1933. doi: 10.1094/PHYTO-09-22-0348-R. Epub 2023 Nov 10. Phytopathology. 2023. PMID: 37261424
-
The genetic basis of local adaptation for pathogenic fungi in agricultural ecosystems.Mol Ecol. 2017 Apr;26(7):2027-2040. doi: 10.1111/mec.13870. Epub 2016 Oct 24. Mol Ecol. 2017. PMID: 27696587 Review.
-
Host Resistance and Temperature-Dependent Evolution of Aggressiveness in the Plant Pathogen Zymoseptoria tritici.Front Microbiol. 2017 Jun 28;8:1217. doi: 10.3389/fmicb.2017.01217. eCollection 2017. Front Microbiol. 2017. PMID: 28702023 Free PMC article.
-
Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen Zymoseptoria tritici (Mycosphaerella graminicola).PLoS Pathog. 2015 Jul 30;11(7):e1005055. doi: 10.1371/journal.ppat.1005055. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26225424 Free PMC article.
-
Local adaptation of plant viruses: lessons from experimental evolution.Mol Ecol. 2017 Apr;26(7):1711-1719. doi: 10.1111/mec.13836. Epub 2016 Sep 22. Mol Ecol. 2017. PMID: 27612225 Review.
Cited by
-
Rapid sequence evolution driven by transposable elements at a virulence locus in a fungal wheat pathogen.BMC Genomics. 2021 May 27;22(1):393. doi: 10.1186/s12864-021-07691-2. BMC Genomics. 2021. PMID: 34044766 Free PMC article.
-
Limited host availability disrupts the genetic correlation between virulence and transmission.Evol Lett. 2023 Jan 31;7(1):58-66. doi: 10.1093/evlett/qrac008. eCollection 2023 Feb 1. Evol Lett. 2023. PMID: 37065437 Free PMC article.
-
Tackling microbial threats in agriculture with integrative imaging and computational approaches.Comput Struct Biotechnol J. 2020 Dec 29;19:372-383. doi: 10.1016/j.csbj.2020.12.018. eCollection 2021. Comput Struct Biotechnol J. 2020. PMID: 33489007 Free PMC article. Review.
-
Combined pangenomics and transcriptomics reveals core and redundant virulence processes in a rapidly evolving fungal plant pathogen.BMC Biol. 2023 Feb 6;21(1):24. doi: 10.1186/s12915-023-01520-6. BMC Biol. 2023. PMID: 36747219 Free PMC article.
-
Population Genomic Evidence for a Repeated Introduction and Rapid Expansion of the Fungal Maize Pathogen Setosphaeria turcica in Europe.Genome Biol Evol. 2023 Aug 1;15(8):evad130. doi: 10.1093/gbe/evad130. Genome Biol Evol. 2023. PMID: 37462319 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

