Impairment of type H vessels by NOX2-mediated endothelial oxidative stress: critical mechanisms and therapeutic targets for bone fragility in streptozotocin-induced type 1 diabetic mice
- PMID: 33664862
- PMCID: PMC7914348
- DOI: 10.7150/thno.50907
Impairment of type H vessels by NOX2-mediated endothelial oxidative stress: critical mechanisms and therapeutic targets for bone fragility in streptozotocin-induced type 1 diabetic mice
Abstract
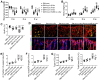
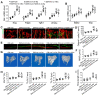
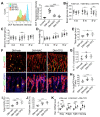
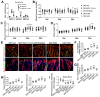
Rationale: Mechanisms underlying the compromised bone formation in type 1 diabetes mellitus (T1DM), which causes bone fragility and frequent fractures, remain poorly understood. Recent advances in organ-specific vascular endothelial cells (ECs) identify type H blood vessel injury in the bone, which actively direct osteogenesis, as a possible player. Methods: T1DM was induced in mice by streptozotocin (STZ) injection in two severity degrees. Bony endothelium, the coupling of angiogenesis and osteogenesis, and bone mass quality were evaluated. Insulin, antioxidants, and NADPH oxidase (NOX) inhibitors were administered to diabetic animals to investigate possible mechanisms and design therapeutic strategies. Results: T1DM in mice led to the holistic abnormality of the vascular system in the bone, especially type H vessels, resulting in the uncoupling of angiogenesis and osteogenesis and inhibition of bone formation. The severity of osteopathy was positively related to glycemic levels. These pathological changes were attenuated by early-started, but not late-started, insulin therapy. ECs in diabetic bones showed significantly higher levels of reactive oxygen species (ROS) and NOX 1 and 2. Impairments of bone vessels and bone mass were effectively ameliorated by treatment with anti-oxidants or NOX2 inhibitors, but not by a NOX1/4 inhibitor. GSK2795039 (GSK), a NOX2 inhibitor, significantly supplemented the insulin effect on the diabetic bone. Conclusions: Diabetic osteopathy could be a chronic microvascular complication of T1DM. The impairment of type H vessels by NOX2-mediated endothelial oxidative stress might be an important contributor that can serve as a therapeutic target for T1DM-induced osteopathy.
Keywords: Diabetic bone fragility; Endothelial damage; NADPH oxidase 2.; Oxidative stress; Type H vessels.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Angiogenesis impairment by the NADPH oxidase-triggered oxidative stress at the bone-implant interface: Critical mechanisms and therapeutic targets for implant failure under hyperglycemic conditions in diabetes.Acta Biomater. 2018 Jun;73:470-487. doi: 10.1016/j.actbio.2018.04.008. Epub 2018 Apr 9. Acta Biomater. 2018. PMID: 29649637
-
Low bone turnover and reduced angiogenesis in streptozotocin-induced osteoporotic mice.Connect Tissue Res. 2016 Jul;57(4):277-89. doi: 10.3109/03008207.2016.1171858. Epub 2016 Mar 30. Connect Tissue Res. 2016. PMID: 27028715
-
MK-4 Ameliorates Diabetic Osteoporosis in Angiogenesis-Dependent Bone Formation by Promoting Mitophagy in Endothelial Cells.Drug Des Devel Ther. 2025 Mar 25;19:2173-2188. doi: 10.2147/DDDT.S503930. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40160965 Free PMC article.
-
Role of miRNA-regulated type H vessel formation in osteoporosis.Front Endocrinol (Lausanne). 2024 May 31;15:1394785. doi: 10.3389/fendo.2024.1394785. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38883597 Free PMC article. Review.
-
Type H blood vessels in bone modeling and remodeling.Theranostics. 2020 Jan 1;10(1):426-436. doi: 10.7150/thno.34126. eCollection 2020. Theranostics. 2020. PMID: 31903130 Free PMC article. Review.
Cited by
-
Aβ -induced excessive mitochondrial fission drives type H blood vessels injury to aggravate bone loss in APP/PS1 mice with Alzheimer's diseases.Aging Cell. 2025 Feb;24(2):e14374. doi: 10.1111/acel.14374. Epub 2024 Oct 16. Aging Cell. 2025. PMID: 39411913 Free PMC article.
-
Region-specific sympatho-adrenergic regulation of specialized vasculature in bone homeostasis and regeneration.iScience. 2023 Jul 22;26(9):107455. doi: 10.1016/j.isci.2023.107455. eCollection 2023 Sep 15. iScience. 2023. PMID: 37680481 Free PMC article.
-
Silicon Enhances Functional Mitochondrial Transfer to Improve Neurovascularization in Diabetic Bone Regeneration.Adv Sci (Weinh). 2025 May;12(19):e2415459. doi: 10.1002/advs.202415459. Epub 2025 Mar 24. Adv Sci (Weinh). 2025. PMID: 40125794 Free PMC article.
-
The Dual Angiogenesis Effects via Nrf2/HO-1 Signaling Pathway of Melatonin Nanocomposite Scaffold on Promoting Diabetic Bone Defect Repair.Int J Nanomedicine. 2024 Mar 16;19:2709-2732. doi: 10.2147/IJN.S449290. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38510794 Free PMC article.
-
Application of extracellular vesicles in diabetic osteoporosis.Front Endocrinol (Lausanne). 2024 Dec 10;15:1466775. doi: 10.3389/fendo.2024.1466775. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39720256 Free PMC article. Review.
References
-
- Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL. et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208–19. - PubMed
-
- Chen SC, Shepherd S, McMillan M, McNeilly J, Foster J, Wong SC. et al. Skeletal Fragility and Its Clinical Determinants in Children With Type 1 Diabetes. J Clin Endocrinol Metab. 2019;104:3585–94. - PubMed
-
- Beckman JA, Creager MA. Vascular Complications of Diabetes. Circ Res. 2016;118:1771–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

