Sunitinib-induced cardiac hypertrophy and the endothelin axis
- PMID: 33664864
- PMCID: PMC7914356
- DOI: 10.7150/thno.49837
Sunitinib-induced cardiac hypertrophy and the endothelin axis
Abstract
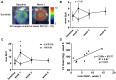
Anti-angiogenics drugs in clinical use for cancer treatment induce cardiotoxic side effects. The endothelin axis is involved in hypertension and cardiac remodelling, and addition of an endothelin receptor antagonist to the anti-angiogenic sunitinib was shown to reduce cardiotoxicity of sunitinib in mice. Here, we explored further the antidote effect of the endothelin receptor antagonist macitentan in sunitinib-treated animals on cardiac remodeling. Methods: Tumor-bearing mice treated per os daily by sunitinib or vehicle were imaged before and after 1, 3 and 6 weeks of treatment by positron emission tomography using [18F]fluorodeoxyglucose and by echocardiography. Non-tumor-bearing animals were randomly assigned to be treated per os daily by vehicle or sunitinib or macitentan or sunitinib+macitentan, and imaged by echocardiography after 5 weeks. Hearts were harvested for histology and molecular analysis at the end of in vivo exploration. Results: Sunitinib treatment increases left ventricular mass and ejection fraction and induces cardiac fibrosis. Sunitinib also induces an early increase in cardiac uptake of [18F]fluorodeoxyglucose, which is significantly correlated with increased left ventricular mass at the end of treatment. Co-administration of macitentan prevents sunitinib-induced hypertension, increase in ejection fraction and cardiac fibrosis, but fails to prevent increase of the left ventricular mass. Conclusion: Early metabolic changes predict sunitinib-induced cardiac remodeling. Endothelin blockade can prevent some but not all cardiotoxic side-effects of sunitinib, in particular left ventricle hypertrophy that appears to be induced by sunitinib through an endothelin-independent mechanism.
Keywords: cardiac hypertrophy; cardiotoxicity; endothelin; metabolic imaging.; sunitinib.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Rini BI, Escudier B, Tomczak P. et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

