The life cycle of voltage-gated Ca2+ channels in neurons: an update on the trafficking of neuronal calcium channels
- PMID: 33664982
- PMCID: PMC7905535
- DOI: 10.1042/NS20200095
The life cycle of voltage-gated Ca2+ channels in neurons: an update on the trafficking of neuronal calcium channels
Abstract
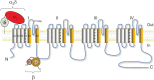
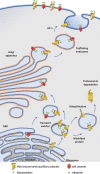
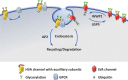
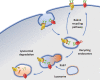
Neuronal voltage-gated Ca2+ (CaV) channels play a critical role in cellular excitability, synaptic transmission, excitation-transcription coupling and activation of intracellular signaling pathways. CaV channels are multiprotein complexes and their functional expression in the plasma membrane involves finely tuned mechanisms, including forward trafficking from the endoplasmic reticulum (ER) to the plasma membrane, endocytosis and recycling. Whether genetic or acquired, alterations and defects in the trafficking of neuronal CaV channels can have severe physiological consequences. In this review, we address the current evidence concerning the regulatory mechanisms which underlie precise control of neuronal CaV channel trafficking and we discuss their potential as therapeutic targets.
Keywords: calcium channel; internalization; trafficking.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Regulation of neuronal excitation-transcription coupling by Kv2.1-induced clustering of somatic L-type Ca2+ channels at ER-PM junctions.Proc Natl Acad Sci U S A. 2021 Nov 16;118(46):e2110094118. doi: 10.1073/pnas.2110094118. Proc Natl Acad Sci U S A. 2021. PMID: 34750263 Free PMC article.
-
Densin-180 Controls the Trafficking and Signaling of L-Type Voltage-Gated Cav1.2 Ca2+ Channels at Excitatory Synapses.J Neurosci. 2017 May 3;37(18):4679-4691. doi: 10.1523/JNEUROSCI.2583-16.2017. Epub 2017 Mar 31. J Neurosci. 2017. PMID: 28363979 Free PMC article.
-
Regulation of high-voltage-activated Ca2+ channel function, trafficking, and membrane stability by auxiliary subunits.Wiley Interdiscip Rev Membr Transp Signal. 2013 Sep 1;2(5):207-220. doi: 10.1002/wmts.93. Wiley Interdiscip Rev Membr Transp Signal. 2013. PMID: 24949251 Free PMC article.
-
Interactions with PDZ proteins diversify voltage-gated calcium channel signaling.J Neurosci Res. 2021 Jan;99(1):332-348. doi: 10.1002/jnr.24650. Epub 2020 Jun 1. J Neurosci Res. 2021. PMID: 32476168 Review.
-
Control of neuronal voltage-gated calcium ion channels from RNA to protein.Trends Neurosci. 2013 Oct;36(10):598-609. doi: 10.1016/j.tins.2013.06.008. Epub 2013 Jul 30. Trends Neurosci. 2013. PMID: 23907011 Free PMC article. Review.
Cited by
-
Functional remodeling of presynaptic voltage-gated calcium channels in superficial layers of the dorsal horn during neuropathic pain.iScience. 2024 May 14;27(6):109973. doi: 10.1016/j.isci.2024.109973. eCollection 2024 Jun 21. iScience. 2024. PMID: 38827405 Free PMC article.
-
SUMOylation of the Kv4.2 Ternary Complex Increases Surface Expression and Current Amplitude by Reducing Internalization in HEK 293 Cells.Front Mol Neurosci. 2021 Nov 2;14:757278. doi: 10.3389/fnmol.2021.757278. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34795560 Free PMC article.
-
CaVβ-subunit dependence of forward and reverse trafficking of CaV1.2 calcium channels.Mol Brain. 2022 May 9;15(1):43. doi: 10.1186/s13041-022-00930-x. Mol Brain. 2022. PMID: 35534894 Free PMC article.
-
Functional and morphological adaptation of medial prefrontal corticotropin releasing factor receptor 1-expressing neurons in male mice following chronic ethanol exposure.Neurobiol Stress. 2024 Jun 17;31:100657. doi: 10.1016/j.ynstr.2024.100657. eCollection 2024 Jul. Neurobiol Stress. 2024. PMID: 38983690 Free PMC article.
-
A molecular complex of Cav1.2/CaMKK2/CaMK1a in caveolae is responsible for vascular remodeling via excitation-transcription coupling.Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2117435119. doi: 10.1073/pnas.2117435119. Epub 2022 Apr 11. Proc Natl Acad Sci U S A. 2022. PMID: 35412911 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

