Osteoclast-derived small extracellular vesicles induce osteogenic differentiation via inhibiting ARHGAP1
- PMID: 33664997
- PMCID: PMC7900016
- DOI: 10.1016/j.omtn.2021.01.031
Osteoclast-derived small extracellular vesicles induce osteogenic differentiation via inhibiting ARHGAP1
Abstract
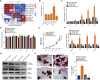
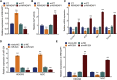
Activated osteoclasts release large amounts of small extracellular vesicles (sEVs) during bone remodeling. However, little is known about whether osteoclast-derived sEVs affect surrounding cells. In this study, osteoclasts were generated by stimulating bone marrow macrophages (BMMs) with macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear actor κB ligand (RANKL). We performed microarray analysis of sEV-microRNAs (miRNAs)s secreted from osteoclast at different stages and identified four miRNAs that were highly expressed in mature osteoclast-derived sEVs. One of these miRNAs, miR-324, significantly induced osteogenic differentiation and mineralization of primary mesenchymal stem cells (MSCs) in vitro by targeting ARHGAP1, a negative regulator of osteogenic differentiation. We next fabricated an sEV-modified scaffold by coating decalcified bone matrix (DBM) with osteoclast-derived sEVs, and the pro-osteogenic regeneration activities of the sEV-modified scaffold were validated in a mouse calvarial defect model. Notably, miR-324-enriched sEV-modified scaffold showed the highest capacity on bone regeneration, whereas inhibition of miR-324 in sEVs abrogated these effects. Taken together, our findings suggest that miR-324-contained sEVs released from mature osteoclast play an essential role in the regulation of osteogenic differentiation and potentially bridge the coupling between osteoclasts and MSCs.
Keywords: extracellular vesicles; osteoclast; osteogenic differentiation.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Osteoclast-derived extracellular miR-106a-5p promotes osteogenic differentiation and facilitates bone defect healing.Cell Signal. 2023 Feb;102:110549. doi: 10.1016/j.cellsig.2022.110549. Epub 2022 Dec 1. Cell Signal. 2023. PMID: 36464103
-
Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration.Tissue Eng Part A. 2021 Jul;27(13-14):962-976. doi: 10.1089/ten.TEA.2020.0141. Epub 2020 Nov 23. Tissue Eng Part A. 2021. PMID: 32962564
-
Mature osteoclast-derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling.J Biol Chem. 2019 Jul 19;294(29):11240-11247. doi: 10.1074/jbc.RA119.007625. Epub 2019 Jun 5. J Biol Chem. 2019. PMID: 31167789 Free PMC article.
-
microRNAs delivered by small extracellular vesicles in MSCs as an emerging tool for bone regeneration.Front Bioeng Biotechnol. 2023 Aug 31;11:1249860. doi: 10.3389/fbioe.2023.1249860. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37720323 Free PMC article. Review.
-
Role of CSF-1 in bone and bone marrow development.Mol Reprod Dev. 1997 Jan;46(1):75-83; discussion 83-4. doi: 10.1002/(SICI)1098-2795(199701)46:1<75::AID-MRD12>3.0.CO;2-2. Mol Reprod Dev. 1997. PMID: 8981367 Review.
Cited by
-
Bibliometric and visualized analysis of the applications of exosomes for bone regeneration.Front Cell Dev Biol. 2025 Mar 17;13:1552727. doi: 10.3389/fcell.2025.1552727. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40166633 Free PMC article.
-
Extracellular vesicles in bone homeostasis: key roles of physiological and pathological conditions.J Bone Miner Metab. 2023 May;41(3):345-357. doi: 10.1007/s00774-022-01362-2. Epub 2022 Aug 9. J Bone Miner Metab. 2023. PMID: 35943593 Review.
-
The effect of bone marrow mesenchymal stem cell-derived extracellular vesicles on bone mineral density and microstructure in osteoporosis: A systematic review and meta-analysis of preclinical studies.PLoS One. 2025 Jun 30;20(6):e0327011. doi: 10.1371/journal.pone.0327011. eCollection 2025. PLoS One. 2025. PMID: 40587472 Free PMC article.
-
Osteoclast-like stromal giant cells in breast cancer likely belong to the spectrum of immunosuppressive tumor-associated macrophages.Front Mol Biosci. 2022 Aug 26;9:894247. doi: 10.3389/fmolb.2022.894247. eCollection 2022. Front Mol Biosci. 2022. PMID: 36090031 Free PMC article.
-
The effects of preosteoblast-derived exosomes on macrophages and bone in mice.J Cell Mol Med. 2024 Jan;28(1):e18029. doi: 10.1111/jcmm.18029. Epub 2023 Nov 6. J Cell Mol Med. 2024. PMID: 37929757 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

