Plasmid encoding microRNA-200c ameliorates periodontitis and systemic inflammation in obese mice
- PMID: 33664998
- PMCID: PMC7899952
- DOI: 10.1016/j.omtn.2021.01.030
Plasmid encoding microRNA-200c ameliorates periodontitis and systemic inflammation in obese mice
Abstract
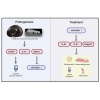
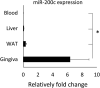
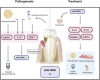
The present study was conducted to characterize microRNA-200c (miR-200c) and its regulators in adipogenic differentiation, obesity, and periodontitis in obese subjects (PiOSs), and to determine the therapeutic efficacy of plasmid DNA encoding miR-200c as a treatment for PiOSs. We report that highly expressed miR-200c in gingival tissues was downregulated in diet-induced obese (DIO) mice and during adipogenic differentiation of human bone marrow mesenchymal stromal cells (hBMSCs). Local injection of Porphyromonas gingivalis lipopolysaccharide (Pg-LPS) in the maxilla interdental gingiva of DIO mice reduced miR-200c in gingival and adipose tissues and induced periodontal inflammation associated with systemic elevation of interleukin-6 (IL-6) and impaired glucose tolerance. The inhibitory functions of Pg-LPS and IL-6 on miR-200c and their effectiveness on Zeb1 were confirmed in vitro. Injection of naked plasmid DNA encoding miR-200c into the gingiva effectively rescued miR-200c downregulation, prevented periodontal and systemic inflammation, and alleviated the impaired glucose metabolism in obese mice with LPS-induced periodontitis. Increased circulating exosomal miR-200c and its function on suppressing proinflammatory cytokines and adipogenesis explained the mechanism(s) of gingival application of miR-200c in attenuating systemic inflammation in PiOSs. These results demonstrated that miR-200c reduced by Pg-LPS and IL-6 in periodontitis and obesity might lead to the pathogenesis of PiOSs, and upregulation of miR-200c in the gingiva presents a therapeutic approach for PiOSs.
Keywords: IL-6; Leptin; Pg-LPS; Zeb1; gene therapy; glucose intolerance; microRNA-200c; mouse; obesity; periodontitis.
© 2021 The Author(s).
Conflict of interest statement
L.H. and B.A.A. have a US patent for miR-200c-based bone regeneration. B.A.A. is the founder of the NaturemiRI Company.
Figures







Similar articles
-
MicroRNA-200c Attenuates Periodontitis by Modulating Proinflammatory and Osteoclastogenic Mediators.Stem Cells Dev. 2019 Aug 1;28(15):1026-1036. doi: 10.1089/scd.2019.0027. Epub 2019 May 20. Stem Cells Dev. 2019. PMID: 31017046 Free PMC article.
-
Topical application of Porphyromonas gingivalis into the gingival pocket in mice leads to chronic‑active infection, periodontitis and systemic inflammation.Int J Mol Med. 2022 Aug;50(2):103. doi: 10.3892/ijmm.2022.5159. Epub 2022 Jun 15. Int J Mol Med. 2022. PMID: 35703359 Free PMC article.
-
The role of long non-coding RNA (lncRNA) nuclear paraspeckle assembly transcript 1 (NEAT1) in chronic periodontitis progression.Bioengineered. 2022 Feb;13(2):2336-2345. doi: 10.1080/21655979.2021.2018387. Bioengineered. 2022. PMID: 35034548 Free PMC article.
-
Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors.Crit Rev Oral Biol Med. 2002;13(2):132-42. doi: 10.1177/154411130201300204. Crit Rev Oral Biol Med. 2002. PMID: 12097356 Review.
-
Biological effects of interleukin-6 on Gingival Fibroblasts: Cytokine regulation in periodontitis.J Cell Physiol. 2018 Sep;233(9):6393-6400. doi: 10.1002/jcp.26521. Epub 2018 Mar 25. J Cell Physiol. 2018. PMID: 29574949 Review.
Cited by
-
Streptococcus gordonii Supragingival Bacterium Oral Infection-Induced Periodontitis and Robust miRNA Expression Kinetics.Int J Mol Sci. 2024 Jun 5;25(11):6217. doi: 10.3390/ijms25116217. Int J Mol Sci. 2024. PMID: 38892405 Free PMC article.
-
Overexpression Effects of miR-424 and BMP2 on the Osteogenesis of Wharton's Jelly-Derived Stem Cells.Biomed Res Int. 2021 Nov 8;2021:7031492. doi: 10.1155/2021/7031492. eCollection 2021. Biomed Res Int. 2021. PMID: 34790821 Free PMC article.
-
Plasmid encoding miRNA-200c delivered by CaCO3-based nanoparticles enhances rat alveolar bone formation.Nanomedicine (Lond). 2022 Aug;17(19):1339-1354. doi: 10.2217/nnm-2022-0151. Epub 2022 Sep 20. Nanomedicine (Lond). 2022. PMID: 36125080 Free PMC article.
-
Emerging Role of MicroRNA-200 Family in Dentistry.Noncoding RNA. 2021 Jun 11;7(2):35. doi: 10.3390/ncrna7020035. Noncoding RNA. 2021. PMID: 34208375 Free PMC article. Review.
-
Human and herpesvirus microRNAs in periodontal disease.Periodontol 2000. 2021 Oct;87(1):325-339. doi: 10.1111/prd.12404. Periodontol 2000. 2021. PMID: 34463985 Free PMC article. Review.
References
-
- Chapple I.L., Bouchard P., Cagetti M.G., Campus G., Carra M.C., Cocco F., Nibali L., Hujoel P., Laine M.L., Lingstrom P. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017;44(Suppl 18):S39–S51. - PubMed
-
- Iwasaki M., Kimura Y., Ogawa H., Yamaga T., Ansai T., Wada T., Sakamoto R., Ishimoto Y., Fujisawa M., Okumiya K. Periodontitis, periodontal inflammation, and mild cognitive impairment: A 5-year cohort study. J. Periodontal Res. 2019;54:233–240. - PubMed
-
- Al-Rawi N.H., Shahid A.M. Oxidative stress, antioxidants, and lipid profile in the serum and saliva of individuals with coronary heart disease: is there a link with periodontal health? Minerva Stomatol. 2017;66:212–225. - PubMed
-
- Benguigui C., Bongard V., Ruidavets J.B., Sixou M., Chamontin B., Ferrières J., Amar J. Evaluation of oral health related to body mass index. Oral Dis. 2012;18:748–755. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

