Dysbiosis of the Saliva Microbiome in Patients With Polycystic Ovary Syndrome
- PMID: 33665172
- PMCID: PMC7921782
- DOI: 10.3389/fcimb.2020.624504
Dysbiosis of the Saliva Microbiome in Patients With Polycystic Ovary Syndrome
Abstract
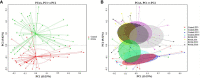
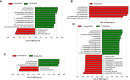
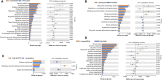
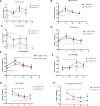
Significant differences in salivary microbiota communities between polycystic ovary syndrome (PCOS) patients and healthy controls have been reported, and interestingly, some salivary microbiota exhibit diurnal oscillation in healthy people. However, whether the diurnal oscillation of salivary microbiota is present in PCOS patients is unknown. In this study, we describe the differences in the saliva microbiome between the PCOS group and the control group at different time points over 24 h. 16S rRNA gene amplicon sequencing was performed on salivary and fecal samples from 10 PCOS patients and 10 healthy controls, and salivary samples were collected at 6-h intervals over 24 h (Zeitgeber (ZT)0, ZT6, ZT12, and ZT18). Among the salivary samples, those from the PCOS group showed significant differences from those of the control group at each time point. Differences were evident in taxa level and metabolic pathways. Interestingly, we found that PCOS disrupted the diurnal rhythm of the salivary microbiota abundance, as determined in the group of healthy women. In addition, no similar changes were found in PCOS patients and controls between the oral and fecal microbiota, including differential microbiota at the phylum level. In this study, significant differences in the composition of the salivary microbiota between PCOS and healthy women were detected at different time points. We also showed that the diurnal rhythm of relative abundance of the salivary microbiota was disrupted in patients with PCOS, which might be related to development of oral-related diseases and systematic metabolic disorders.
Keywords: 16S rRNA; diurnal rhythm; fecal microbiota; polycystic ovary syndrome; salivary microbiome.
Copyright © 2021 Li, Li, Qian, Liu, Cao, Ma, He, Chen, Geng and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Characterization of the gut microbiota in polycystic ovary syndrome with dyslipidemia.BMC Microbiol. 2024 May 17;24(1):169. doi: 10.1186/s12866-024-03329-x. BMC Microbiol. 2024. PMID: 38760705 Free PMC article.
-
Dysbiotic alteration in the fecal microbiota of patients with polycystic ovary syndrome.Microbiol Spectr. 2024 Aug 6;12(8):e0429123. doi: 10.1128/spectrum.04291-23. Epub 2024 Jul 11. Microbiol Spectr. 2024. PMID: 38990031 Free PMC article.
-
Changes in Vaginal Microbiome Diversity in Women With Polycystic Ovary Syndrome.Front Cell Infect Microbiol. 2021 Nov 3;11:755741. doi: 10.3389/fcimb.2021.755741. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34804995 Free PMC article.
-
Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: a systematic review and meta-analysis.BMC Med. 2023 Aug 9;21(1):302. doi: 10.1186/s12916-023-02975-8. BMC Med. 2023. PMID: 37559119 Free PMC article.
-
Gut Microbiota in Patients with Polycystic Ovary Syndrome: a Systematic Review.Reprod Sci. 2022 Jan;29(1):69-83. doi: 10.1007/s43032-020-00430-0. Epub 2021 Jan 6. Reprod Sci. 2022. PMID: 33409871
Cited by
-
Bacterial Vaginosis (BV) and Vaginal Microbiome Disorders in Women Suffering from Polycystic Ovary Syndrome (PCOS).Diagnostics (Basel). 2024 Feb 12;14(4):404. doi: 10.3390/diagnostics14040404. Diagnostics (Basel). 2024. PMID: 38396443 Free PMC article.
-
Probiotics and Polycystic Ovary Syndrome: A Perspective for Management in Adolescents with Obesity.Nutrients. 2023 Jul 14;15(14):3144. doi: 10.3390/nu15143144. Nutrients. 2023. PMID: 37513562 Free PMC article. Review.
-
Bidirectional association between polycystic ovary syndrome and periodontal diseases.Front Endocrinol (Lausanne). 2023 Jan 23;14:1008675. doi: 10.3389/fendo.2023.1008675. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36755917 Free PMC article. Review.
-
A Potential Link Between Oral Microbiota and Female Reproductive Health.Microorganisms. 2025 Mar 7;13(3):619. doi: 10.3390/microorganisms13030619. Microorganisms. 2025. PMID: 40142512 Free PMC article. Review.
-
Polycystic Ovary Syndrome and Endometrial Cancer: A Scoping Review of the Literature on Gut Microbiota.Cells. 2022 Sep 28;11(19):3038. doi: 10.3390/cells11193038. Cells. 2022. PMID: 36231000 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

