Genes Regulating Spermatogenesis and Sperm Function Associated With Rare Disorders
- PMID: 33665191
- PMCID: PMC7921155
- DOI: 10.3389/fcell.2021.634536
Genes Regulating Spermatogenesis and Sperm Function Associated With Rare Disorders
Abstract
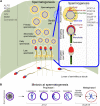
Spermatogenesis is a cell differentiation process that ensures the production of fertilizing sperm, which ultimately fuse with an egg to form a zygote. Normal spermatogenesis relies on Sertoli cells, which preserve cell junctions while providing nutrients for mitosis and meiosis of male germ cells. Several genes regulate normal spermatogenesis, some of which are not exclusively expressed in the testis and control multiple physiological processes in an organism. Loss-of-function mutations in some of these genes result in spermatogenesis and sperm functionality defects, potentially leading to the insurgence of rare genetic disorders. To identify genetic intersections between spermatogenesis and rare diseases, we screened public archives of human genetic conditions available on the Genetic and Rare Diseases Information Center (GARD), the Online Mendelian Inheritance in Man (OMIM), and the Clinical Variant (ClinVar), and after an extensive literature search, we identified 22 distinct genes associated with 21 rare genetic conditions and defective spermatogenesis or sperm function. These protein-coding genes regulate Sertoli cell development and function during spermatogenesis, checkpoint signaling pathways at meiosis, cellular organization and shape definition during spermiogenesis, sperm motility, and capacitation at fertilization. A number of these genes regulate folliculogenesis and oogenesis as well. For each gene, we review the genotype-phenotype association together with associative or causative polymorphisms in humans, and provide a description of the shared molecular mechanisms that regulate gametogenesis and fertilization obtained in transgenic animal models.
Keywords: Sertoli; acrosome; centrosome; genetic disease; infertility; sperm motility.
Copyright © 2021 Linn, Ghanem, Bhakta, Greer and Avella.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Alazami A. M., Al-Saif A., Al-Semari A., Bohlega S., Zlitni S., Alzahrani F., et al. (2008). Mutations in C2orf37, encoding a nucleolar protein, cause hypogonadism, alopecia, diabetes mellitus, mental retardation, and extrapyramidal syndrome. Am. J. Hum. Genet. 83 684–691. 10.1016/j.ajhg.2008.10.018 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

