Spatial survey of non-collagenous proteins in mineralizing and non-mineralizing vertebrate tissues ex vivo
- PMID: 33665237
- PMCID: PMC7900015
- DOI: 10.1016/j.bonr.2021.100754
Spatial survey of non-collagenous proteins in mineralizing and non-mineralizing vertebrate tissues ex vivo
Abstract
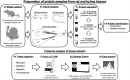
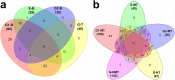
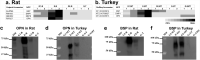
Bone biomineralization is a complex process in which type I collagen and associated non-collagenous proteins (NCPs), including glycoproteins and proteoglycans, interact closely with inorganic calcium and phosphate ions to control the precipitation of nanosized, non-stoichiometric hydroxyapatite (HAP, idealized stoichiometry Ca10(PO4)6(OH)2) within the organic matrix of a tissue. The ability of certain vertebrate tissues to mineralize is critically related to several aspects of their function. The goal of this study was to identify specific NCPs in mineralizing and non-mineralizing tissues of two animal models, rat and turkey, and to determine whether some NCPs are unique to each type of tissue. The tissues investigated were rat femur (mineralizing) and tail tendon (non-mineralizing) and turkey leg tendon (having both mineralizing and non-mineralizing regions in the same individual specimen). An experimental approach ex vivo was designed for this investigation by combining sequential protein extraction with comprehensive protein mapping using proteomics and Western blotting. The extraction method enabled separation of various NCPs based on their association with either the extracellular organic collagenous matrix phases or the inorganic mineral phases of the tissues. The proteomics work generated a complete picture of NCPs in different tissues and animal species. Subsequently, Western blotting provided validation for some of the proteomics findings. The survey then yielded generalized results relevant to various protein families, rather than only individual NCPs. This study focused primarily on the NCPs belonging to the small leucine-rich proteoglycan (SLRP) family and the small integrin-binding ligand N-linked glycoproteins (SIBLINGs). SLRPs were found to be associated only with the collagenous matrix, a result suggesting that they are mainly involved in structural matrix organization and not in mineralization. SIBLINGs as well as matrix Gla (γ-carboxyglutamate) protein were strictly localized within the inorganic mineral phase of mineralizing tissues, a finding suggesting that their roles are limited to mineralization. The results from this study indicated that osteocalcin was closely involved in mineralization but did not preclude possible additional roles as a hormone. This report provides for the first time a spatial survey and comparison of NCPs from mineralizing and non-mineralizing tissues ex vivo and defines the proteome of turkey leg tendons as a model for vertebrate mineralization.
Keywords: B, rat bone; BSP, bone sialoprotein; DCN, decorin; E, EDTA extract; ECM, extracellular matrix; G, guanidine-HCl-only extract (for non-mineralizing tissues); G1, first guanidine-HCl extract; G2, second guanidine-HCl extract; Gla, gamma-carboxylated glutamic acid; MGP, matrix Gla protein; MT, turkey mineralizing tendon; Mineralization; NCP, non-collagenous protein; NMT, turkey never-mineralizing tendon; NT, turkey not-yet-mineralized tendon; Non-collagenous protein; OCN, osteocalcin; OPN, osteopontin; Proteomics; SIBLING, small integrin-binding ligand N-linked glycoprotein; SLRP, small leucine-rich proteoglycan; T, rat tail tendon; TLT, turkey leg tendon (gastrocnemius); TNAP, tissue-nonspecific alkaline phosphatase; Type I collagen; Vertebrate.
© 2021 Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Addison W.N., Azari F., Sorensen E.S., Kaartinen M.T., McKee M.D. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 2007;282(21):15872–15883. - PubMed
-
- Baht G.S., Hunter G.K., Goldberg H.A. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008;27(7):600–608. - PubMed
-
- Berthet-Colominas C., Miller A., White S.W. Structural study of the calcifying collagen in turkey leg tendons. J. Mol. Biol. 1979;134(3):431–445. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

