Polydopamine/poly(sulfobetaine methacrylate) Co-deposition coatings triggered by CuSO4/H2O2 on implants for improved surface hemocompatibility and antibacterial activity
- PMID: 33665495
- PMCID: PMC7887402
- DOI: 10.1016/j.bioactmat.2021.01.025
Polydopamine/poly(sulfobetaine methacrylate) Co-deposition coatings triggered by CuSO4/H2O2 on implants for improved surface hemocompatibility and antibacterial activity
Erratum in
-
Corrigendum to "Polydopamine/poly(sulfobetaine methacrylate) Co-deposition coatings triggered by CuSO4/H2O2 on implants for improved surface hemocompatibility and antibacterial activity" [ Bioactive materials 6 (2021) 2546-2556].Bioact Mater. 2022 Feb 12;17:195-196. doi: 10.1016/j.bioactmat.2022.01.037. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35386459 Free PMC article.
Abstract
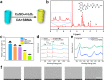
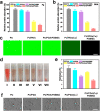
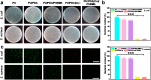
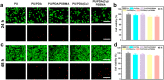
Implanted biomaterials such as medical catheters are prone to be adhered by proteins, platelets and bacteria due to their surface hydrophobicity characteristics, and then induce related infections and thrombosis. Hence, the development of a versatile strategy to endow surfaces with antibacterial and antifouling functions is particularly significant for blood-contacting materials. In this work, CuSO4/H2O2 was used to trigger polydopamine (PDA) and poly-(sulfobetaine methacrylate) (PSBMA) co-deposition process to endow polyurethane (PU) antibacterial and antifouling surface (PU/PDA(Cu)/PSBMA). The zwitterions contained in the PU/PDA(Cu)/PSBMA coating can significantly improve surface wettability to reduce protein adsorption, thereby improving its blood compatibility. In addition, the copper ions released from the metal-phenolic networks (MPNs) imparted them more than 90% antibacterial activity against E. coli and S. aureus. Notably, PU/PDA(Cu)/PSBMA also exhibits excellent performance in vivo mouse catheter-related infections models. Thus, the PU/PDA(Cu)/PSBMA has great application potential for developing multifunctional surface coatings for blood-contacting materials so as to improve antibacterial and anticoagulant properties.
Keywords: Antibacterial; Copper ions; Hemocompatibility; Surface modification; Zwitterionic polymer.
© 2021 [The Author/The Authors].
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Noimark S., Dunnill C.W., Wilson M., Parkin I.P. The role of surfaces in catheter-associated infections. Chem. Soc. Rev. 2009;38(12):3435–3448. - PubMed
-
- Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16(7):397–409. - PubMed
-
- Parienti J.-J., Mongardon N., Megarbane B., Mira J.-P., Kalfon P., Gros A., Marque S., Thuong M., Pottier V., Ramakers M., Savary B., Seguin A., Valette X., Terzi N., Sauneuf B., Cattoir V., Mermel L.A., du Cheyron D., Grp S.S. Intravascular complications of central venous catheterization by insertion site. N. Engl. J. Med. 2015;373(13):1220–1229. - PubMed
-
- Gbyli R., Mercaldi A., Sundaram H., Amoako K.A. Achieving totally local anticoagulation on blood contacting devices. Advanced Materials Interfaces. 2018;5(4):1700954.
-
- Gorbet M.B., Sefton M.V. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25(26):5681–5703. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

