Selective postnatal excitation of neocortical pyramidal neurons results in distinctive behavioral and circuit deficits in adulthood
- PMID: 33665575
- PMCID: PMC7907816
- DOI: 10.1016/j.isci.2021.102157
Selective postnatal excitation of neocortical pyramidal neurons results in distinctive behavioral and circuit deficits in adulthood
Abstract
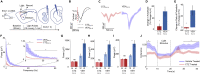
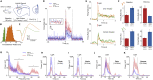
In genetic and pharmacological models of neurodevelopmental disorders, and human data, neural activity is altered within the developing neocortical network. This commonality begs the question of whether early enhancement in excitation might be a common driver, across etiologies, of characteristic behaviors. We tested this concept by chemogenetically driving cortical pyramidal neurons during postnatal days 4-14. Hyperexcitation of Emx1-, but not dopamine transporter-, parvalbumin-, or Dlx5/6-expressing neurons, led to decreased social interaction and increased grooming activity in adult animals. In vivo optogenetic interrogation in adults revealed decreased baseline but increased stimulus-evoked firing rates of pyramidal neurons and impaired recruitment of inhibitory neurons. Slice recordings in adults from prefrontal cortex layer 5 pyramidal neurons revealed decreased intrinsic excitability and increased synaptic E/I ratio. Together these results support the prediction that enhanced pyramidal firing during development, in otherwise normal cortex, can selectively drive altered adult circuit function and maladaptive changes in behavior.
Keywords: Behavioral Neuroscience; Cellular Neuroscience; Developmental Neuroscience.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Backman C.M., Malik N., Zhang Y., Shan L., Grinberg A., Hoffer B.J., Westphal H., Tomac A.C. Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

