Cell of origin in biliary tract cancers and clinical implications
- PMID: 33665585
- PMCID: PMC7902553
- DOI: 10.1016/j.jhepr.2021.100226
Cell of origin in biliary tract cancers and clinical implications
Abstract
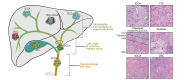
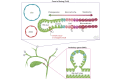
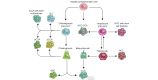
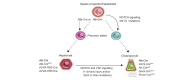
Biliary tract cancers (BTCs) are aggressive epithelial malignancies that can arise at any point of the biliary tree. Albeit rare, their incidence and mortality rates have been rising steadily over the past 40 years, highlighting the need to improve current diagnostic and therapeutic strategies. BTCs show high inter- and intra-tumour heterogeneity both at the morphological and molecular level. Such complex heterogeneity poses a substantial obstacle to effective interventions. It is widely accepted that the observed heterogeneity may be the result of a complex interplay of different elements, including risk factors, distinct molecular alterations and multiple potential cells of origin. The use of genetic lineage tracing systems in experimental models has identified cholangiocytes, hepatocytes and/or progenitor-like cells as the cells of origin of BTCs. Genomic evidence in support of the distinct cell of origin hypotheses is growing. In this review, we focus on recent advances in the histopathological subtyping of BTCs, discuss current genomic evidence and outline lineage tracing studies that have contributed to the current knowledge surrounding the cell of origin of these tumours.
Keywords: ARID1A, AT-rich interactive domain-containing protein 1A; BAP1, BRCA1-associated protein 1; BRAF, v-Raf murine sarcoma viral oncogene homolog B; BTC, biliary tract cancer; Biliary tract cancers; CCA, cholangiocarcinoma; CDKN2A/B, cyclin-dependent kinase inhibitor 2A/B; CK, cytokeratin; CLC, cholangiolocarcinoma; Cell of origin; Cholangiocarcinoma; CoH, Canal of Hering; DCR, disease control rate; ER, estrogen receptor; ERBB2/3, Erb-B2 Receptor Tyrosine Kinase 2/3; FGFR, fibroblast growth factor receptor; FGFR2, Fibroblast Growth Factor Receptor 2; GBC, gallbladder cancer; GEMM, genetically engineered mouse models; Genomics; HCC, hepatocellular carcinoma; HPCs, hepatic progenitor cells; IDH, isocitrate dehydrogenase; KRAS, Kirsten Rat Sarcoma Viral Oncogene Homolog; Lineage tracing; MET, Hepatocyte Growth Factor Receptor; MST1, Macrophage Stimulating 1; NA, not applicable; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NGS, next-generation sequencing; NR, not reported; NTRK, Neurotrophic Receptor Tyrosine Kinase 1; ORR, objective response rate; OS, overall survival; PBG, peribiliary gland; PFS, progression- free survival; PIK3CA, Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha; PLC, primary liver cancer; PRKACA/B, Protein Kinase CAMP-Activated Catalytic Subunit Alpha/Beta; PROM1, Prominin 1; PSC, primary sclerosing cholangitis; Personalized therapy; RNF43, Ring Finger Protein 43; SMAD4, SMAD Family Member 4; TBG, thyroid binding globulin; TP53, Tumor Protein P53; WHO, World Health Organization; dCCA, distal cholangiocarcinoma; eCCA, extrahepatic cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma; mo, months; pCCA, perihilar cholangiocarcinoma.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
References
-
- Bertuccio P., Malvezzi M., Carioli G., Hashim D., Boffetta P., El-Serag H.B. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104–114. - PubMed
-
- Khan S.A., Tavolari S., Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39:19–31. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

