Where are the children in national hepatitis C policies? A global review of national strategic plans and guidelines
- PMID: 33665586
- PMCID: PMC7898178
- DOI: 10.1016/j.jhepr.2021.100227
Where are the children in national hepatitis C policies? A global review of national strategic plans and guidelines
Abstract
Background & aims: It is estimated that 3.26 million children and adolescents worldwide have chronic HCV infection. To date, the global response has focused on the adult population, but direct-acting antiviral (DAA) regimens are now approved for children aged ≥3 years. This global review describes the current status of policies on HCV testing and treatment in children, adolescents, and pregnant women in WHO Member States.
Methods: We identified national strategic plans and/or clinical practice guidelines (CPGs) for HCV infection from a World Health Organization (WHO) database of national policies from Member States as of August 2019. A standardised proforma was used to abstract data on polices or recommendations on testing and treatment in children, adolescents and pregnant women. Analysis was stratified according to the country-income status and results were validated through WHO regional focal points through August 2020.
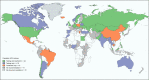
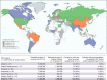
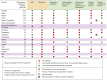
Results: National HCV policies were available for 122 of the 194 WHO Member States. Of these, the majority (n = 71/122, 58%) contained no policy recommendations for either testing or treatment in children or adolescents. Of the 51 countries with policies, 24 had specific policies for both testing and treatment, and were mainly from the European region; 18 countries for HCV testing only (12 from high- or upper-middle income); and 9 countries for treatment only (7 high- or upper-middle income). Twenty-one countries provided specific treatment recommendations: 13 recommended DAA-based regimens for adolescents ≥12 years and 6 still recommended interferon/ribavirin-based regimens.
Conclusions: There are significant gaps in policies for HCV-infected children and adolescents. Updated guidance on testing and treatment with newly approved DAA regimens for younger age groups is needed, especially in most affected countries.
Lay summary: To date, the predominant focus of the global response towards elimination of hepatitis C has been on the testing and treatment of adults. Much less attention has been paid to testing and treatment among children and adolescents, although in 2018 an estimated 3.26 million were infected with HCV. Our review shows that many countries have no national guidance on HCV testing and treatment in children and adolescents. It highlights the urgent need for advocacy and updated policies and guidelines specific for children and adolescents.
Keywords: AASLD, American Association for the Study of Liver Diseases; APASL, Asian Pacific Association for the Study of the Liver; Adolescents; CPGs, clinical practice guidelines; Children; Clinical practice guidelines; DAAs, direct-acting antivirals; EASL, European Association for the Study of the Liver; ESPGHAN, European Society for Paediatric Gastroenterology Hepatology and Nutrition; GHSS, Global Health Sector Strategy; GLE, glecaprevir; GT, genotype; Hepatitis C; IDU, injecting drug use; IFN, interferon; LED, ledipasvir; LMICs, low- and middle-income countries; MoH, ministries of health; NASPGHAN, North American Society for Pediatric Gastroenterology Hepatology and Nutrition; NSPs, national strategic plans; National strategic plans; PIB, pibrentasvir; Policies; Policy review; Pregnancy; RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir; WHO, World Health Organization.
© 2021 The Author(s).
Conflict of interest statement
FM, AM, PC, HB, and PE declare no competing interests. CT has previously received grant funding from 10.13039/100010877ViiV Healthcare and BMS (through Penta Foundation). IJC reports grants from 10.13039/100006483Abbvie, 10.13039/100008021Bristol Myers Squibb, 10.13039/100016016Gilead, 10.13039/100008897Janssen Pharmaceuticals, and 10.13039/100010877ViiV Healthcare (through the PENTA Foundation). Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
References
-
- World Health Organization . WHO; Geneva: 2017. Global hepatitis report.https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-en... Available from:
-
- World Health Organization . WHO; Geneva: 2019. Progress report on HIV, viral hepatitis and sexually transmitted infections.https://apps.who.int/iris/bitstream/handle/10665/324797/WHO-CDS-HIV-19.7... 2019. Available from:
-
- World Health Organization . WHO; Geneva: 2016. Global health sector strategy on viral hepatitis 2016-2021.https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-... Available from:
-
- World Health Organization Regional Office for South-East Asia . WHO; New Delhi: 2016. Regional action plan for viral hepatitis in South-East Asia: 2016-2021.https://apps.who.int/iris/bitstream/handle/10665/258735/9789290225386-en... Available from:
-
- Pan American Health Organization . PAHO; Washington, DC: 2016. Plan of Action for the prevention and control of Viral Hepatitis.https://www.paho.org/hq/dmdocuments/2016/2016-cha-plan-action-prev-hep-e... Available from:
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous