Bile acid-receptor TGR5 deficiency worsens liver injury in alcohol-fed mice by inducing intestinal microbiota dysbiosis
- PMID: 33665587
- PMCID: PMC7903352
- DOI: 10.1016/j.jhepr.2021.100230
Bile acid-receptor TGR5 deficiency worsens liver injury in alcohol-fed mice by inducing intestinal microbiota dysbiosis
Abstract
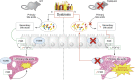
Background & aims: Bile-acid metabolism and the intestinal microbiota are impaired in alcohol-related liver disease. Activation of the bile-acid receptor TGR5 (or GPBAR1) controls both biliary homeostasis and inflammatory processes. We examined the role of TGR5 in alcohol-induced liver injury in mice.
Methods: We used TGR5-deficient (TGR5-KO) and wild-type (WT) female mice, fed alcohol or not, to study the involvement of liver macrophages, the intestinal microbiota (16S sequencing), and bile-acid profiles (high-performance liquid chromatography coupled to tandem mass spectrometry). Hepatic triglyceride accumulation and inflammatory processes were assessed in parallel.
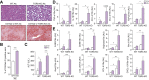
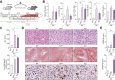
Results: TGR5 deficiency worsened liver injury, as shown by greater steatosis and inflammation than in WT mice. Isolation of liver macrophages from WT and TGR5-KO alcohol-fed mice showed that TGR5 deficiency did not increase the pro-inflammatory phenotype of liver macrophages but increased their recruitment to the liver. TGR5 deficiency induced dysbiosis, independently of alcohol intake, and transplantation of the TGR5-KO intestinal microbiota to WT mice was sufficient to worsen alcohol-induced liver inflammation. Secondary bile-acid levels were markedly lower in alcohol-fed TGR5-KO than normally fed WT and TGR5-KO mice. Consistent with these results, predictive analysis showed the abundance of bacterial genes involved in bile-acid transformation to be lower in alcohol-fed TGR5-KO than WT mice. This altered bile-acid profile may explain, in particular, why bile-acid synthesis was not repressed and inflammatory processes were exacerbated.
Conclusions: A lack of TGR5 was associated with worsening of alcohol-induced liver injury, a phenotype mainly related to intestinal microbiota dysbiosis and an altered bile-acid profile, following the consumption of alcohol.
Lay summary: Excessive chronic alcohol intake can induce liver disease. Bile acids are molecules produced by the liver and can modulate disease severity. We addressed the specific role of TGR5, a bile-acid receptor. We found that TGR5 deficiency worsened alcohol-induced liver injury and induced both intestinal microbiota dysbiosis and bile-acid pool remodelling. Our data suggest that both the intestinal microbiota and TGR5 may be targeted in the context of human alcohol-induced liver injury.
Keywords: ALD, alcohol-related liver diseases; ALT, alanine aminotransferase; Alc, alcohol; Alcoholic liver disease; BA, bile acids; BHI, brain heart infusion; Bile acid; C57, conventional mice; C57C57, conventional mice transplanted with their own IM; CA, cholic acid; CCL, CC motif chemokine ligands; CDCA, chenodeoxycholic acid; Col1a1, collagen type-I alpha-1 chain; DCA, deoxycholic acid; Dysbiosis; FDR, false-discovery rate; FXR, farnesoid X receptor; Gut-liver axis; IM, intestinal microbiota; Inflammation; KC, Kupffer cells; KO, knockout; Kupffer cells; LCA, lithocholic acid; LDA, linear discriminative analysis; LEfsE, LDA effect size; MCA, muricholic acid; MO, monocytes/macrophages; Microbiome; NFkB, nuclear factor-kappa B; OTU, operational taxonomic unit; PCA, principal component analysis; PCoA, principal coordinate analysis; PICRUSt, phylogenetic investigation of communities by reconstruction of unobserved states; RIN, RNA integrity number; TBA, total bile acids; TG, triglycerides; TGF, transforming growth factor; TIMP1, tissue inhibitor of metalloproteinase 1; TNF, tumour necrosis factor; UDCA, ursodeoxycholic acid; WT, wild-type; WTKO, WT mice transplanted with the IM of TGR5-KO mice; alpha-SMA, alpha-smooth muscle actin; mMMP9, matrix metallopeptidase 9.
© 2021 The Author(s).
Conflict of interest statement
During the last 3 years: AMC has received travel grants from Biocodex and royalties from Elsevier-Masson, John Libbey Eurotext, Solar, and Flammarion/Versilio; GP has received travel funds from Abbvie, Biocodex, Gilead, and MSD, consulting fees from Adare, Biocodex, Gilead, Pilèje, and Servier, and royalties from Elsevier-Masson, John Libbey Eurotext, Solar, and Flammarion/Versilio; DC has received travel grants from Biocodex and Gilead, and royalties from John Libbey Eurotext. All other authors declare no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures






References
-
- Llopis M., Cassard A.M., Wrzosek L., Boschat L., Bruneau A., Ferrere G. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65:830–839. - PubMed
-
- Wrzosek L., Ciocan D., Hugot C., Spatz M., Dupeux M., Houron C. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut. 2020 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

