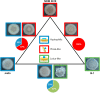
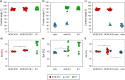
Topography quantifications allow for identifying the contribution of parental strains to physical properties of co-cultured biofilms
- PMID: 33665611
- PMCID: PMC7902895
- DOI: 10.1016/j.bioflm.2021.100044
Topography quantifications allow for identifying the contribution of parental strains to physical properties of co-cultured biofilms
Abstract
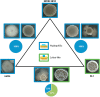
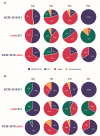
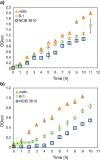
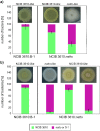
Most biofilm research has so far focused on investigating biofilms generated by single bacterial strains. However, such single-species biofilms are rare in nature where bacteria typically coexist with other microorganisms. Although, from a biological view, the possible interactions occurring between different bacteria are well studied, little is known about what determines the material properties of a multi-species biofilm. Here, we ask how the co-cultivation of two B. subtilis strains affects certain important biofilm properties such as surface topography and wetting behavior. We find that, even though each daughter colony typically resembles one of the parent colonies in terms of morphology and wetting, it nevertheless exhibits a significantly different surface topography. Yet, this difference is only detectable via a quantitative metrological analysis of the biofilm surface. Furthermore, we show that this difference is due to the presence of bacteria belonging to the 'other' parent strain, which does not dominate the biofilm features. The findings presented here may pinpoint new strategies for how biofilms with hybrid properties could be generated from two different bacterial strains. In such engineered biofilms, it might be possible to combine desired properties from two strains by co-cultivation.
Keywords: Biofilms; Computational image classification; Feature detection; Synergy; Topography; Wetting behavior.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Surface topology affects wetting behavior of Bacillus subtilis biofilms.NPJ Biofilms Microbiomes. 2017 Apr 25;3:11. doi: 10.1038/s41522-017-0018-1. eCollection 2017. NPJ Biofilms Microbiomes. 2017. PMID: 28649412 Free PMC article.
-
A Dual-Species Biofilm with Emergent Mechanical and Protective Properties.J Bacteriol. 2019 Aug 22;201(18):e00670-18. doi: 10.1128/JB.00670-18. Print 2019 Sep 15. J Bacteriol. 2019. PMID: 30833350 Free PMC article.
-
Topography and Expansion Patterns at the Biofilm-Agar Interface in Bacillus subtilis Biofilms.Microorganisms. 2020 Dec 31;9(1):84. doi: 10.3390/microorganisms9010084. Microorganisms. 2020. PMID: 33396528 Free PMC article.
-
Evolved Biofilm: Review on the Experimental Evolution Studies of Bacillus subtilis Pellicles.J Mol Biol. 2019 Nov 22;431(23):4749-4759. doi: 10.1016/j.jmb.2019.02.005. Epub 2019 Feb 12. J Mol Biol. 2019. PMID: 30769118 Review.
-
Hydrodynamics and surface properties influence biofilm proliferation.Adv Colloid Interface Sci. 2021 Feb;288:102336. doi: 10.1016/j.cis.2020.102336. Epub 2020 Dec 10. Adv Colloid Interface Sci. 2021. PMID: 33421727 Review.
Cited by
-
Vertical growth dynamics of biofilms.Proc Natl Acad Sci U S A. 2023 Mar 14;120(11):e2214211120. doi: 10.1073/pnas.2214211120. Epub 2023 Mar 7. Proc Natl Acad Sci U S A. 2023. PMID: 36881625 Free PMC article.
-
Tilt-induced polar order and topological defects in growing bacterial populations.PNAS Nexus. 2022 Dec 21;1(5):pgac269. doi: 10.1093/pnasnexus/pgac269. eCollection 2022 Nov. PNAS Nexus. 2022. PMID: 36712383 Free PMC article.
References
-
- Edmunds M. On the association between Myrmarachne spp. (Salticidae) and ants. Bull Br Arachnol Soc. 1978;4:149–160.
-
- Wang S., Jiang L. Definition of superhydrophobic states. Adv Mater. 2007;19:3423–3424. doi: 10.1002/adma.200700934. - DOI
-
- Shim M.H., Kim J., Park C.H. The effects of surface energy and roughness on the hydrophobicity of woven fabrics. Textil Res J. 2014;84:1268–1278. doi: 10.1177/0040517513495945. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

