Exploring the role of BAFF as biomarker in the detection of uveal melanoma metastases
- PMID: 33665679
- PMCID: PMC11801909
- DOI: 10.1007/s00432-021-03555-0
Exploring the role of BAFF as biomarker in the detection of uveal melanoma metastases
Abstract
Purpose: While B-cell activating factor (BAFF) was identified to promote the invasion in other malignancies, its role in the progression of uveal melanoma (UM) still remains unexplored. Here, we analysed the serum level of BAFF in UM patients with regard to its significance as biomarker for the metastases.
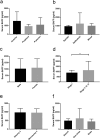
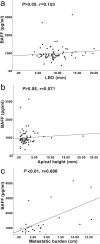
Methods: In this retrospective study, serum BAFF levels in 173 UM patients (36 with metastases and 137 without), and 23 healthy controls were measured with a multiplexed sandwich ELISA system and then correlated with clinicopathological characteristics such as primary tumor size, tumor location, histological cell type, sex, cancer stage, cytogenetic alterations of chromosome 3, and the metastatic burden. Immunohistochemical staining of 50 UM tissue specimens was also performed to evaluate the expression of BAFF and its receptors BAFF-R and TACI.
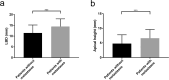
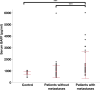
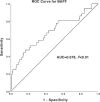
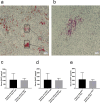
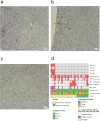
Results: The metastatic patients were identified to have significantly higher serum BAFF levels (mean ± SD, 1520.8 ± 1182.1 pg/ml) than those without metastases (950.4 ± 494.6 pg/ml) and controls (810.3 ± 140.5 pg/ml). While no distinctions were detected with regard to tumor location, histological cell type, gender, and monosomy 3, the patients in cancer stages II, III, and IV displayed higher serum BAFF levels than those in stage I. The serum BAFF level was significantly correlated with the metastatic burden. The serum BAFF level of 1120 pg/ml was identified to have the best performance for distinguishing the metastatic patients from non-metastatic patients. In the kinetic study, we noticed that 20.8% of the analysed patients already demonstrated elevated serum BAFF concentrations before the clinical diagnosis of metastases. Positive BAFF staining was detected in the cytoplasm of single tumor cells (in 13 specimens), macrophages (in 12 specimens), and tumor-infiltrating lymphocytes (TILs) (in 13 specimens). The expressions of BAFF-R and TACI were also observed in 17 and 36 of the 50 tested UM specimens, respectively.
Conclusions: Our study first suggests that BAFF might be a promising serum marker for the detection of UM metastases.
Keywords: B-cell activating factor; Metastasis; Serum markers; Tumor microenvironment; Tumor-infiltrating immune cells; Uveal melanoma.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures
Similar articles
-
Malignant Transformation of Ocular Melanocytoma to Uveal Melanoma: A Clinicopathologic Review of Three Novel Unsuspected Cases of a Likely Under-Recognized Phenomenon, With Review of the Literature and Molecular Genetic Data.Am J Ophthalmol. 2025 Aug;276:126-145. doi: 10.1016/j.ajo.2025.03.044. Epub 2025 Mar 31. Am J Ophthalmol. 2025. PMID: 40174716 Review.
-
DJ-1: a promising marker in metastatic uveal melanoma.J Cancer Res Clin Oncol. 2015 Feb;141(2):315-21. doi: 10.1007/s00432-014-1804-2. Epub 2014 Aug 17. J Cancer Res Clin Oncol. 2015. PMID: 25129821 Free PMC article.
-
Differential expression of p52 and RelB proteins in the metastatic and non-metastatic groups of uveal melanoma with patient outcome.J Cancer Res Clin Oncol. 2019 Dec;145(12):2969-2982. doi: 10.1007/s00432-019-03052-5. Epub 2019 Oct 14. J Cancer Res Clin Oncol. 2019. PMID: 31612319 Free PMC article.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Prognostic value of PRAME expression in uveal melanoma: a meta-analysis.J Clin Pathol. 2025 Jul 18;78(8):519-526. doi: 10.1136/jcp-2024-210032. J Clin Pathol. 2025. PMID: 40295098
Cited by
-
HOOK1 Inhibits the Progression of Renal Cell Carcinoma via TGF-β and TNFSF13B/VEGF-A Axis.Adv Sci (Weinh). 2023 Jun;10(17):e2206955. doi: 10.1002/advs.202206955. Epub 2023 Apr 21. Adv Sci (Weinh). 2023. PMID: 37085921 Free PMC article.
-
Evaluation of a Three-Marker Panel for the Detection of Uveal Melanoma Metastases: A Single-Center Retrospective Analysis.Cancers (Basel). 2021 May 18;13(10):2464. doi: 10.3390/cancers13102464. Cancers (Basel). 2021. PMID: 34070192 Free PMC article.
-
The BAFF-APRIL System in Cancer.Cancers (Basel). 2023 Mar 16;15(6):1791. doi: 10.3390/cancers15061791. Cancers (Basel). 2023. PMID: 36980677 Free PMC article. Review.
-
Exploring the causal relationship between immune cell characteristics and melanoma: A two-way Mendelian randomization study.Medicine (Baltimore). 2025 Jun 13;104(24):e42888. doi: 10.1097/MD.0000000000042888. Medicine (Baltimore). 2025. PMID: 40527765 Free PMC article.
-
A two-step, two-sample Mendelian randomization analysis investigating the interplay between gut microbiota, immune cells, and melanoma skin cancer.Medicine (Baltimore). 2024 Nov 8;103(45):e40432. doi: 10.1097/MD.0000000000040432. Medicine (Baltimore). 2024. PMID: 39533622 Free PMC article.
References
-
- Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (2017) AJCC cancer staging manual. Springer, Switzerland
-
- Bakalian S, Marshall J-C, Logan P, Faingold D, Maloney S, di Cesare S, Martins C, Fernandes BF, Burnier MN (2008) Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res 14:951 - PubMed
-
- Barak V, Kaiserman I, Frenkel S, Hendler K, Kalickman I, Pe’Er J (2011) The dynamics of serum tumor markers in predicting metastatic uveal melanoma (part 1). Anticancer Res 31:345–349 - PubMed
-
- Berus T, Halon A, Markiewicz A, Orlowska-Heitzman J, Romanowska-Dixon B, Donizy P (2017) Clinical, histopathological and cytogenetic prognosticators in uveal melanoma—a comprehensive review. Anticancer Res 37:6541–6549 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

