Bispecific VH/Fab antibodies targeting neutralizing and non-neutralizing Spike epitopes demonstrate enhanced potency against SARS-CoV-2
- PMID: 33666135
- PMCID: PMC7939556
- DOI: 10.1080/19420862.2021.1893426
Bispecific VH/Fab antibodies targeting neutralizing and non-neutralizing Spike epitopes demonstrate enhanced potency against SARS-CoV-2
Abstract
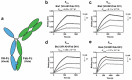
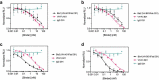
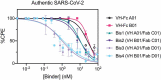
Numerous neutralizing antibodies that target SARS-CoV-2 have been reported, and most directly block binding of the viral Spike receptor-binding domain (RBD) to angiotensin-converting enzyme II (ACE2). Here, we deliberately exploit non-neutralizing RBD antibodies, showing they can dramatically assist in neutralization when linked to neutralizing binders. We identified antigen-binding fragments (Fabs) by phage display that bind RBD, but do not block ACE2 or neutralize virus as IgGs. When these non-neutralizing Fabs were assembled into bispecific VH/Fab IgGs with a neutralizing VH domain, we observed a ~ 25-fold potency improvement in neutralizing SARS-CoV-2 compared to the mono-specific bi-valent VH-Fc alone or the cocktail of the VH-Fc and IgG. This effect was epitope-dependent, reflecting the unique geometry of the bispecific antibody toward Spike. Our results show that a bispecific antibody that combines both neutralizing and non-neutralizing epitopes on Spike-RBD is a promising and rapid engineering strategy to improve the potency of SARS-CoV-2 antibodies.
Keywords: Bispecific; COVID-19; SARS-CoV-2; knob-in-hole; neutralizing antibody.
Figures

Similar articles
-
Neutralizing Human Antibodies against Severe Acute Respiratory Syndrome Coronavirus 2 Isolated from a Human Synthetic Fab Phage Display Library.Int J Mol Sci. 2021 Feb 15;22(4):1913. doi: 10.3390/ijms22041913. Int J Mol Sci. 2021. PMID: 33671877 Free PMC article.
-
Bi-paratopic and multivalent VH domains block ACE2 binding and neutralize SARS-CoV-2.Nat Chem Biol. 2021 Jan;17(1):113-121. doi: 10.1038/s41589-020-00679-1. Epub 2020 Oct 20. Nat Chem Biol. 2021. PMID: 33082574 Free PMC article.
-
Structural Basis of a Human Neutralizing Antibody Specific to the SARS-CoV-2 Spike Protein Receptor-Binding Domain.Microbiol Spectr. 2021 Oct 31;9(2):e0135221. doi: 10.1128/Spectrum.01352-21. Epub 2021 Oct 13. Microbiol Spectr. 2021. PMID: 34643438 Free PMC article.
-
Analysis of the molecular mechanism of SARS-CoV-2 antibodies.Biochem Biophys Res Commun. 2021 Aug 20;566:45-52. doi: 10.1016/j.bbrc.2021.06.001. Epub 2021 Jun 5. Biochem Biophys Res Commun. 2021. PMID: 34116356 Free PMC article. Review.
-
Recognition of the SARS-CoV-2 receptor binding domain by neutralizing antibodies.Biochem Biophys Res Commun. 2021 Jan 29;538:192-203. doi: 10.1016/j.bbrc.2020.10.012. Epub 2020 Oct 10. Biochem Biophys Res Commun. 2021. PMID: 33069360 Free PMC article. Review.
Cited by
-
Overcoming antibody-resistant SARS-CoV-2 variants with bispecific antibodies constructed using non-neutralizing antibodies.iScience. 2024 Feb 29;27(4):109363. doi: 10.1016/j.isci.2024.109363. eCollection 2024 Apr 19. iScience. 2024. PMID: 38500835 Free PMC article.
-
Antibody Phage Display Technology for Sensor-Based Virus Detection: Current Status and Future Prospects.Biosensors (Basel). 2023 Jun 9;13(6):640. doi: 10.3390/bios13060640. Biosensors (Basel). 2023. PMID: 37367005 Free PMC article. Review.
-
Facilitating high throughput bispecific antibody production and potential applications within biopharmaceutical discovery workflows.MAbs. 2024 Jan-Dec;16(1):2311992. doi: 10.1080/19420862.2024.2311992. Epub 2024 Feb 21. MAbs. 2024. PMID: 39674918 Free PMC article. Review.
-
Hexamerization of Anti-SARS CoV IgG1 Antibodies Improves Neutralization Capacity.Front Immunol. 2022 May 4;13:864775. doi: 10.3389/fimmu.2022.864775. eCollection 2022. Front Immunol. 2022. PMID: 35603164 Free PMC article.
-
A Candidate Therapeutic Monoclonal Antibody Inhibits Both HRSV and HMPV Replication in Mice.Biomedicines. 2022 Oct 8;10(10):2516. doi: 10.3390/biomedicines10102516. Biomedicines. 2022. PMID: 36289776 Free PMC article.
References
-
- Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, Kunze KL, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-2 month experience. medRxiv. 2020. doi:10.1101/2020.08.12.20169359. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
