Systematic functional analysis of rab GTPases reveals limits of neuronal robustness to environmental challenges in flies
- PMID: 33666175
- PMCID: PMC8016483
- DOI: 10.7554/eLife.59594
Systematic functional analysis of rab GTPases reveals limits of neuronal robustness to environmental challenges in flies
Abstract
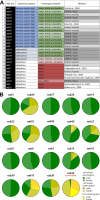
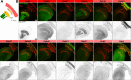
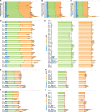
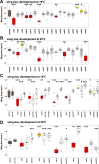
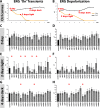
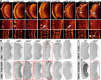
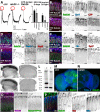
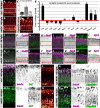
Rab GTPases are molecular switches that regulate membrane trafficking in all cells. Neurons have particular demands on membrane trafficking and express numerous Rab GTPases of unknown function. Here, we report the generation and characterization of molecularly defined null mutants for all 26 rab genes in Drosophila. In flies, all rab genes are expressed in the nervous system where at least half exhibit particularly high levels compared to other tissues. Surprisingly, loss of any of these 13 nervous system-enriched Rabs yielded viable and fertile flies without obvious morphological defects. However, all 13 mutants differentially affected development when challenged with different temperatures, or neuronal function when challenged with continuous stimulation. We identified a synaptic maintenance defect following continuous stimulation for six mutants, including an autophagy-independent role of rab26. The complete mutant collection generated in this study provides a basis for further comprehensive studies of Rab GTPases during development and function in vivo.
Keywords: D. melanogaster; Drosophila; Rab GTPase; cell biology; mutant collection; neuroscience.
© 2021, Kohrs et al.
Conflict of interest statement
FK, ID, BP, EJ, FK, SL, FP, HW, TM, GL, CC, MB No competing interests declared, PH Reviewing editor, eLife
Figures








References
-
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, Hoskins RA, Spradling AC. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. - DOI - PMC - PubMed
-
- Chan CC, Scoggin S, Wang D, Cherry S, Dembo T, Greenberg B, Jin EJ, Kuey C, Lopez A, Mehta SQ, Perkins TJ, Brankatschk M, Rothenfluh A, Buszczak M, Hiesinger PR. Systematic discovery of rab GTPases with synaptic functions in Drosophila. Current Biology. 2011;21:1704–1715. doi: 10.1016/j.cub.2011.08.058. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

