In vitro activity of itraconazole against SARS-CoV-2
- PMID: 33666253
- PMCID: PMC8014624
- DOI: 10.1002/jmv.26917
In vitro activity of itraconazole against SARS-CoV-2
Abstract
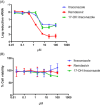
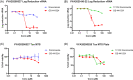
Although vaccination campaigns are currently being rolled out to prevent coronavirus disease (COVID-19), antivirals will remain an important adjunct to vaccination. Antivirals against coronaviruses do not exist, hence global drug repurposing efforts have been carried out to identify agents that may provide clinical benefit to patients with COVID-19. Itraconazole, an antifungal agent, has been reported to have activity against animal coronaviruses. Using cell-based phenotypic assays, the in vitro antiviral activity of itraconazole and 17-OH itraconazole was assessed against clinical isolates from a German and Belgian patient infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Itraconazole demonstrated antiviral activity in human Caco-2 cells (EC50 = 2.3 µM; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay). Similarly, its primary metabolite, 17-OH itraconazole, showed inhibition of SARS-CoV-2 activity (EC50 = 3.6 µM). Remdesivir inhibited viral replication with an EC50 = 0.4 µM. Itraconazole and 17-OH itraconazole resulted in a viral yield reduction in vitro of approximately 2-log10 and approximately 1-log10 , as measured in both Caco-2 cells and VeroE6-eGFP cells, respectively. The viral yield reduction brought about by remdesivir or GS-441524 (parent nucleoside of the antiviral prodrug remdesivir; positive control) was more pronounced, with an approximately 3-log10 drop and >4-log10 drop in Caco-2 cells and VeroE6-eGFP cells, respectively. Itraconazole and 17-OH itraconazole exert in vitro low micromolar activity against SARS-CoV-2. Despite the in vitro antiviral activity, itraconazole did not result in a beneficial effect in hospitalized COVID-19 patients in a clinical study (EudraCT Number: 2020-001243-15).
Keywords: 17-OH itraconazole; Caco-2 cells; SARS-CoV-2; VeroE6-eGFP cells; in vitro; itraconazole.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Sandra De Meyer, Ellen Van Damme, Christophe Buyck, and Marnix Van Loock are employees and may be stock owners of Johnson & Johnson. Sandra Ciesek received research funding from Janssen for this study. Steven De Jonghe, Dirk Jochmans, Pieter Leyssen, and Johan Neyts are employees from KU Leuven and received funding from Janssen for this study.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

