Limited specificity of commercially available SARS-CoV-2 IgG ELISAs in serum samples of African origin
- PMID: 33666297
- PMCID: PMC8014856
- DOI: 10.1111/tmi.13569
Limited specificity of commercially available SARS-CoV-2 IgG ELISAs in serum samples of African origin
Abstract
Objectives: Specific serological tests are mandatory for reliable SARS-CoV-2 diagnostics and seroprevalence studies. Here, we assess the specificities of four commercially available SARS-CoV-2 IgG ELISAs in serum/plasma panels originating from Africa, South America, and Europe.
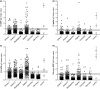
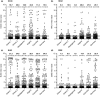
Methods: 882 serum/plasma samples collected from symptom-free donors before the COVID-19 pandemic in three African countries (Ghana, Madagascar, Nigeria), Colombia, and Germany were analysed with three nucleocapsid-based ELISAs (Euroimmun Anti-SARS-CoV-2-NCP IgG, EDI™ Novel Coronavirus COVID-19 IgG, Mikrogen recomWell SARS-CoV-2 IgG), one spike/S1-based ELISA (Euroimmun Anti-SARS-CoV-2 IgG), and in-house common cold CoV ELISAs.
Results: High specificity was confirmed for all SARS-CoV-2 IgG ELISAs for Madagascan (93.4-99.4%), Colombian (97.8-100.0%), and German (95.9-100.0%) samples. In contrast, specificity was much lower for the Ghanaian and Nigerian serum panels (Ghana: NCP-based assays 77.7-89.7%, spike/S1-based assay 94.3%; Nigeria: NCP-based assays 39.3-82.7%, spike/S1-based assay 90.7%). 15 of 600 African sera were concordantly classified as positive in both the NCP-based and the spike/S1-based Euroimmun ELISA, but did not inhibit spike/ACE2 binding in a surrogate virus neutralisation test. IgG antibodies elicited by previous infections with common cold CoVs were found in all sample panels, including those from Madagascar, Colombia, and Germany and thus do not inevitably hamper assay specificity. Nevertheless, high levels of IgG antibodies interacting with OC43 NCP were found in all 15 SARS-CoV-2 NCP/spike/S1 ELISA positive sera.
Conclusions: Depending on the chosen antigen and assay protocol, SARS-CoV-2 IgG ELISA specificity may be significantly reduced in certain populations probably due to interference of immune responses to endemic pathogens like other viruses or parasites.
Keywords: Africa; Enzyme-Linked Immunosorbent Assay; SARS-CoV-2; immunoglobulin G; seroepidemiologic studies; specificity.
© 2021 The Authors Tropical Medicine & International Health Published by John Wiley & Sons Ltd.
Figures


References
-
- Studies ACfS . https://africacenter.org/spotlight/mapping‐risk‐factors‐spread‐covid‐19‐.... 2020.
-
- WHO . COVID‐19 Weekly Epidemiological Update, 16 Feb 2021.
-
- Mbow M, Lell B, Jochems SP et al. COVID‐19 in Africa: Dampening the storm? Science 2020: 369: 624–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 01KI20210/German Federal Ministry of Education and Research
- GU 883/4-1/German Research Foundation (DFG)
- GU 883/5-1/German Research Foundation (DFG)
- ZMV I1-2517WHO005/German Federal Ministry of Health through support of the WHO Collaborating Centre for Arboviruses and Hemorrhagic Fever Viruses at BNITM
- ZMV I1-2517GHP-704/German Federal Ministry of Health through support of the WHO Collaborating Centre for Arboviruses and Hemorrhagic Fever Viruses at BNITM
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

