Azobioisosteres of Curcumin with Pronounced Activity against Amyloid Aggregation, Intracellular Oxidative Stress, and Neuroinflammation
- PMID: 33666306
- PMCID: PMC8048673
- DOI: 10.1002/chem.202005263
Azobioisosteres of Curcumin with Pronounced Activity against Amyloid Aggregation, Intracellular Oxidative Stress, and Neuroinflammation
Abstract
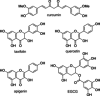
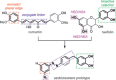
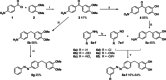
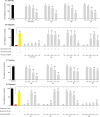
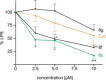
Many (poly-)phenolic natural products, for example, curcumin and taxifolin, have been studied for their activity against specific hallmarks of neurodegeneration, such as amyloid-β 42 (Aβ42) aggregation and neuroinflammation. Due to their drawbacks, arising from poor pharmacokinetics, rapid metabolism, and even instability in aqueous medium, the biological activity of azobenzene compounds carrying a pharmacophoric catechol group, which have been designed as bioisoteres of curcumin has been examined. Molecular simulations reveal the ability of these compounds to form a hydrophobic cluster with Aβ42, which adopts different folds, affecting the propensity to populate fibril-like conformations. Furthermore, the curcumin bioisosteres exceeded the parent compound in activity against Aβ42 aggregation inhibition, glutamate-induced intracellular oxidative stress in HT22 cells, and neuroinflammation in microglial BV-2 cells. The most active compound prevented apoptosis of HT22 cells at a concentration of 2.5 μm (83 % cell survival), whereas curcumin only showed very low protection at 10 μm (21 % cell survival).
Keywords: amyloid beta; bioisosterism; natural products; neuroprotectivity; replica-exchange molecular dynamics.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- E. Babusikova, A. Evinova, J. Jurecekova, M. Jesenak, D. Dobrota in Advanced Understanding of Neurodegenerative Diseases, IntechOpen, 2011.
-
- None
-
- Glenner G. G., Wong C. W., Biochem. Biophys. Res. Commun. 1984, 120, 885–890; - PubMed
-
- Haass C., Selkoe D. J., Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

