Maternal systemic vascular dysfunction in a primate model of defective uterine spiral artery remodeling
- PMID: 33666502
- PMCID: PMC8260378
- DOI: 10.1152/ajpheart.00613.2020
Maternal systemic vascular dysfunction in a primate model of defective uterine spiral artery remodeling
Abstract
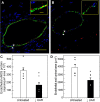
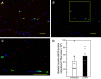
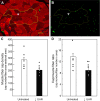
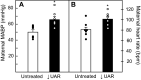
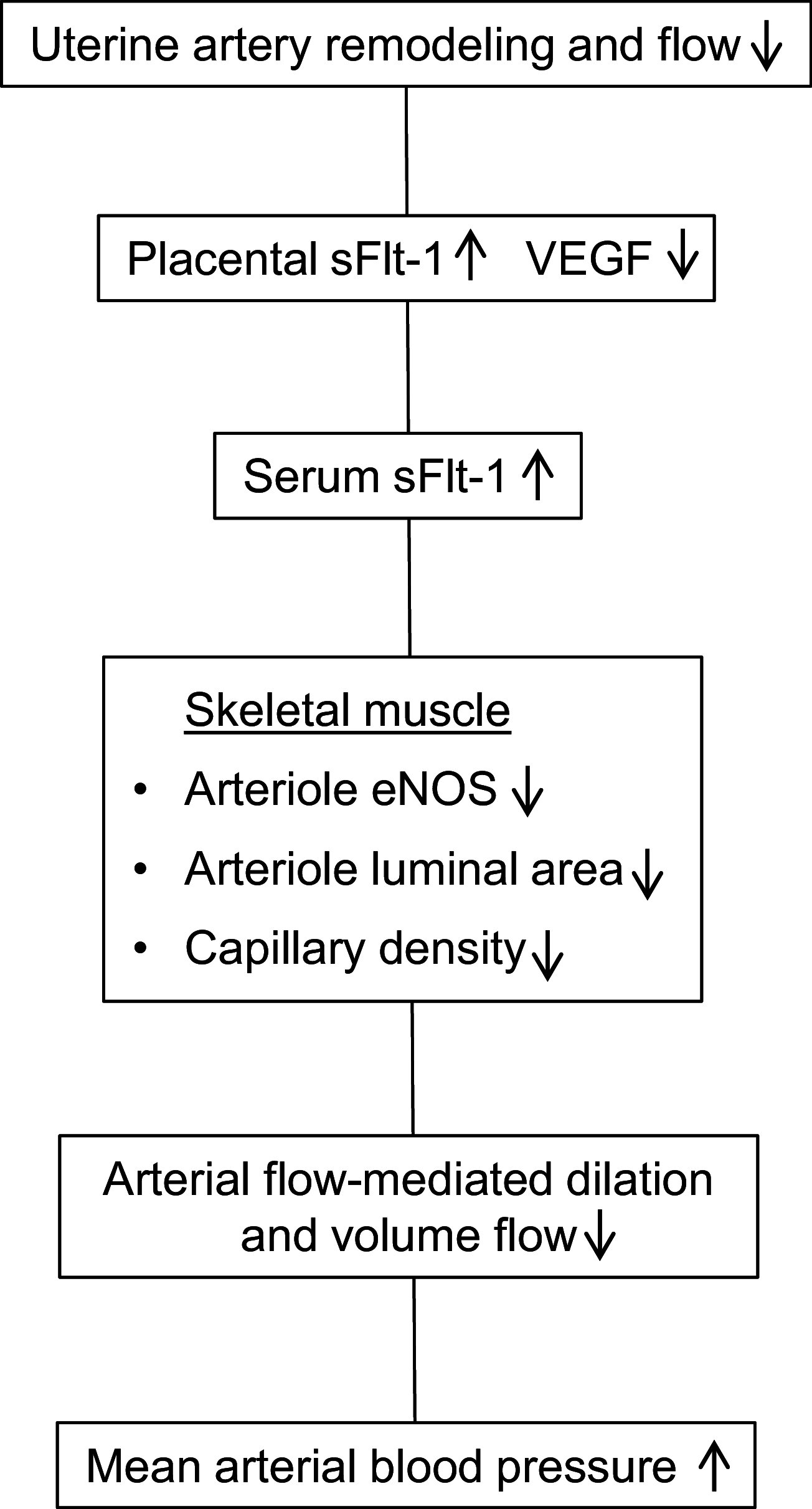
Uterine spiral artery remodeling (UAR) is essential for placental perfusion and fetal development. A defect in UAR underpins placental ischemia disorders, e.g., preeclampsia, that result in maternal systemic vascular endothelial dysfunction and hypertension. We have established a model of impaired UAR by prematurely elevating maternal serum estradiol levels during the first trimester of baboon pregnancy. However, it is unknown whether this experimental paradigm is associated with maternal vascular endothelial dysfunction. Therefore, in the present study baboons were administered estradiol on days 25-59 of gestation to suppress UAR and maternal vascular function determined on day 165 (term = 184 days) peripherally and in skeletal muscle, which accounts for over 40% of body mass and 25% of resting systemic vascular resistance. Maternal serum sFlt-1 levels were 2.5-fold higher (P < 0.05), and skeletal muscle arteriolar endothelial nitric oxide synthase (eNOS) protein expression and luminal area, and skeletal muscle capillary density were 30-50% lower (P < 0.05) in UAR suppressed baboons. Coinciding with these changes in eNOS expression, luminal area, and capillary density, maternal brachial artery flow-mediated dilation and volume flow were 70% and 55% lower (P < 0.05), respectively, and mean arterial blood pressure 29% higher (P < 0.01) in UAR defective baboons. In summary, maternal vascular function was disrupted in a baboon model of impaired UAR. These results highlight the translational impact of this primate model and relevance to adverse conditions of human pregnancy underpinned by improper uterine artery transformation.NEW & NOTEWORTHY Maternal vascular dysfunction is a hallmark of abnormal human pregnancy, particularly early-onset preeclampsia, elicited by impaired UAR. The present study makes the novel discovery that maternal systemic vascular dysfunction was induced in a baboon experimental model of impaired UAR. This study highlights the translational relevance of this nonhuman primate model to adverse conditions of human pregnancy underpinned by defective UAR.
Keywords: artery; primate; remodeling; uterine; vascular.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
Placental sFlt-1 Gene Delivery in Early Primate Pregnancy Suppresses Uterine Spiral Artery Remodeling.Endocrinology. 2022 Apr 1;163(4):bqac012. doi: 10.1210/endocr/bqac012. Endocrinology. 2022. PMID: 35134145 Free PMC article.
-
Vascular Endothelial Growth Factor Delivery to Placental Basal Plate Promotes Uterine Artery Remodeling in the Primate.Endocrinology. 2019 Jun 1;160(6):1492-1505. doi: 10.1210/en.2019-00059. Endocrinology. 2019. PMID: 31002314 Free PMC article.
-
Prematurely elevating estradiol in early baboon pregnancy suppresses uterine artery remodeling and expression of extravillous placental vascular endothelial growth factor and α1β1 and α5β1 integrins.Endocrinology. 2012 Jun;153(6):2897-906. doi: 10.1210/en.2012-1141. Epub 2012 Apr 11. Endocrinology. 2012. PMID: 22495671 Free PMC article.
-
Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia.Am J Physiol Heart Circ Physiol. 2020 Sep 1;319(3):H661-H681. doi: 10.1152/ajpheart.00202.2020. Epub 2020 Aug 7. Am J Physiol Heart Circ Physiol. 2020. PMID: 32762557 Free PMC article. Review.
-
Regulation of Uterine Spiral Artery Remodeling: a Review.Reprod Sci. 2020 Oct;27(10):1932-1942. doi: 10.1007/s43032-020-00212-8. Epub 2020 Jun 16. Reprod Sci. 2020. PMID: 32548805 Free PMC article. Review.
Cited by
-
Maternal urinary bisphenols and phthalates in relation to estimated fetal weight across mid to late pregnancy.Environ Int. 2023 Apr;174:107922. doi: 10.1016/j.envint.2023.107922. Epub 2023 Apr 10. Environ Int. 2023. PMID: 37075581 Free PMC article.
-
Placental sFlt-1 Gene Delivery in Early Primate Pregnancy Suppresses Uterine Spiral Artery Remodeling.Endocrinology. 2022 Apr 1;163(4):bqac012. doi: 10.1210/endocr/bqac012. Endocrinology. 2022. PMID: 35134145 Free PMC article.
-
B-flow/spatiotemporal image correlation M-mode: novel ultrasound method that detects decrease in spiral artery luminal diameter in first trimester in primate model of impaired spiral artery remodeling.Ultrasound Obstet Gynecol. 2022 Mar;59(3):358-364. doi: 10.1002/uog.23753. Ultrasound Obstet Gynecol. 2022. PMID: 34358371 Free PMC article.
-
Placental growth factor mediates pathological uterine angiogenesis by activating the NFAT5-SGK1 signaling axis in the endometrium: implications for preeclampsia development.Biol Res. 2024 Aug 17;57(1):55. doi: 10.1186/s40659-024-00526-w. Biol Res. 2024. PMID: 39152497 Free PMC article.
-
Placental Ischemia Says "NO" to Proper NOS-Mediated Control of Vascular Tone and Blood Pressure in Preeclampsia.Int J Mol Sci. 2021 Oct 19;22(20):11261. doi: 10.3390/ijms222011261. Int J Mol Sci. 2021. PMID: 34681920 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

