Fine particulate matter (PM2.5) inhalation-induced alterations in the plasma lipidome as promoters of vascular inflammation and insulin resistance
- PMID: 33666505
- PMCID: PMC8163652
- DOI: 10.1152/ajpheart.00881.2020
Fine particulate matter (PM2.5) inhalation-induced alterations in the plasma lipidome as promoters of vascular inflammation and insulin resistance
Abstract
Fine particulate matter (PM2.5) air pollution exposure increases the risk of developing cardiovascular disease (CVD). Although the precise mechanisms by which air pollution exposure increases CVD risk remain uncertain, research indicates that PM2.5-induced endothelial dysfunction contributes to CVD risk. Previous studies demonstrate that concentrated ambient PM2.5 (CAP) exposure induces vascular inflammation and impairs insulin and vascular endothelial growth factor (VEGF) signaling dependent on pulmonary oxidative stress. To assess whether CAP exposure induces these vascular effects via plasmatic factors, we incubated aortas from naïve mice with plasma isolated from mice exposed to HEPA-filtered air or CAP (9 days) and examined vascular inflammation and insulin and VEGF signaling. We found that treatment of naïve aortas with plasma from CAP-exposed mice activates NF-κBα and induces insulin and VEGF resistance, indicating transmission by plasmatic factor(s). To identify putative factors, we exposed lung-specific ecSOD-transgenic (ecSOD-Tg) mice and wild-type (WT) littermates to CAP at concentrations of either ∼60 µg/m3 (CAP60) or ∼100 µg/m3 (CAP100) and measured the abundance of plasma metabolites by mass spectrometry. In WT mice, both CAP concentrations increased levels of fatty acids such as palmitate, myristate, and palmitoleate and decreased numerous phospholipid species; however, these CAP-induced changes in the plasma lipidome were prevented in ecSOD-Tg mice. Consistent with the literature, we found that fatty acids such as palmitate are sufficient to promote endothelial inflammation. Collectively, our findings suggest that PM2.5 exposure, by inducing pulmonary oxidative stress, promotes unique lipidomic changes characterized by high levels of circulating fatty acids, which are sufficient to trigger vascular pathology.NEW & NOTEWORTHY We found that circulating plasma constituents are responsible for air pollution-induced vascular pathologies. Inhalation of fine particulate matter (≤PM2.5) promotes a unique form of dyslipidemia that manifests in a manner dependent upon pulmonary oxidative stress. The air pollution-engendered dyslipidemic phenotype is characterized by elevated free fatty acid species and diminished phospholipid species, which could contribute to vascular inflammation and loss of insulin sensitivity.
Keywords: air pollution; cardiovascular disease; free fatty acids; plasma metabolome; pulmonary oxidative stress.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

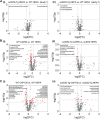

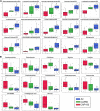
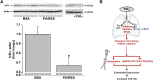
Comment in
-
Reply to Della Guardia and Shin.Am J Physiol Heart Circ Physiol. 2022 Jun 1;322(6):H973-H974. doi: 10.1152/ajpheart.00186.2022. Am J Physiol Heart Circ Physiol. 2022. PMID: 35481792 No abstract available.
-
The role of adipose tissue dysfunction in PM2.5-induced vascular pathology.Am J Physiol Heart Circ Physiol. 2022 Jun 1;322(6):H971-H972. doi: 10.1152/ajpheart.00156.2022. Am J Physiol Heart Circ Physiol. 2022. PMID: 35481793 No abstract available.
References
-
- World Health Organization (WHO). https://www.who.int/health-topics/air-pollution#tab=tab_1.
-
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010. doi: 10.1161/CIR.0b013e3181dbece1. - DOI - PubMed
-
- Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 109: 71–77, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

