Microfluidic electrical impedance assessment of red blood cell-mediated microvascular occlusion
- PMID: 33666615
- PMCID: PMC8170703
- DOI: 10.1039/d0lc01133a
Microfluidic electrical impedance assessment of red blood cell-mediated microvascular occlusion
Abstract
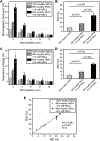
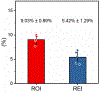
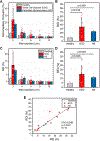
Alterations in the deformability of red blood cells (RBCs), occurring in hemolytic blood disorders such as sickle cell disease (SCD), contribute to vaso-occlusion and disease pathophysiology. There are few functional in vitro assays for standardized assessment of RBC-mediated microvascular occlusion. Here, we present the design, fabrication, and clinical testing of the Microfluidic Impedance Red Cell Assay (MIRCA) with embedded capillary network-based micropillar arrays and integrated electrical impedance measurement electrodes to address this need. The micropillar arrays consist of microcapillaries ranging from 12 μm to 3 μm, with each array paired with two sputtered gold electrodes to measure the impedance change of the array before and after sample perfusion through the microfluidic device. We define RBC occlusion index (ROI) and RBC electrical impedance index (REI), which represent the cumulative percentage occlusion and cumulative percentage impedance change, respectively. We demonstrate the promise of MIRCA in two common red cell disorders, SCD and hereditary spherocytosis. We show that the electrical impedance measurement reflects the microvascular occlusion, where REI significantly correlates with ROI that is obtained via high-resolution microscopy imaging of the microcapillary arrays. Further, we show that RBC-mediated microvascular occlusion, represented by ROI and REI, associates with clinical treatment outcomes and correlates with in vivo hemolytic biomarkers, lactate dehydrogenase (LDH) level and absolute reticulocyte count (ARC) in SCD. Impedance measurement obviates the need for high-resolution imaging, enabling future translation of this technology for widespread access, portable and point-of-care use. Our findings suggest that the presented microfluidic design and the integrated electrical impedance measurement provide a reproducible functional test for standardized assessment of RBC-mediated microvascular occlusion. MIRCA and the newly defined REI may serve as an in vitro therapeutic efficacy benchmark for assessing the clinical outcome of emerging RBC-modifying targeted and curative therapies.
Conflict of interest statement
CONFLICT OF INTEREST
A patent application has been filed by Case Western Reserve University for this technology.
Figures


References
-
- Lacelle PL, Semin Hematol, 1970, 7, 355–371. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

