Group 2 innate lymphoid cells support hematopoietic recovery under stress conditions
- PMID: 33666647
- PMCID: PMC7941180
- DOI: 10.1084/jem.20200817
Group 2 innate lymphoid cells support hematopoietic recovery under stress conditions
Abstract
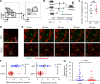
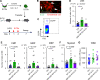
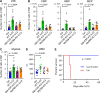
The cell-cycle status of hematopoietic stem and progenitor cells (HSPCs) becomes activated following chemotherapy-induced stress, promoting bone marrow (BM) regeneration; however, the underlying molecular mechanism remains elusive. Here we show that BM-resident group 2 innate lymphoid cells (ILC2s) support the recovery of HSPCs from 5-fluorouracil (5-FU)-induced stress by secreting granulocyte-macrophage colony-stimulating factor (GM-CSF). Mechanistically, IL-33 released from chemo-sensitive B cell progenitors activates MyD88-mediated secretion of GM-CSF in ILC2, suggesting the existence of a B cell-ILC2 axis for maintaining hematopoietic homeostasis. GM-CSF knockout mice treated with 5-FU showed severe loss of myeloid lineage cells, causing lethality, which was rescued by transferring BM ILC2s from wild-type mice. Further, the adoptive transfer of ILC2s to 5-FU-treated mice accelerates hematopoietic recovery, while the reduction of ILC2s results in the opposite effect. Thus, ILC2s may function by "sensing" the damaged BM spaces and subsequently support hematopoietic recovery under stress conditions.
© 2021 Sudo et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures










Similar articles
-
Group 2 innate lymphoid cells in bone marrow regulate osteoclastogenesis in a reciprocal manner via RANKL, GM-CSF and IL-13.Int Immunol. 2021 Oct 29;33(11):573-585. doi: 10.1093/intimm/dxab062. Int Immunol. 2021. PMID: 34498703
-
Delineating the effects of 5-fluorouracil and follicle-stimulating hormone on mouse bone marrow stem/progenitor cells.Stem Cell Res Ther. 2016 Apr 19;7(1):59. doi: 10.1186/s13287-016-0311-6. Stem Cell Res Ther. 2016. PMID: 27095238 Free PMC article.
-
Bone marrow connexin-43 expression is critical for hematopoietic regeneration after chemotherapy.Cell Commun Adhes. 2005 Jul-Dec;12(5-6):307-17. doi: 10.1080/15419060500514200. Cell Commun Adhes. 2005. PMID: 16531325
-
Endothelial Cell-Selective Adhesion Molecule Expression in Hematopoietic Stem/Progenitor Cells Is Essential for Erythropoiesis Recovery after Bone Marrow Injury.PLoS One. 2016 Apr 25;11(4):e0154189. doi: 10.1371/journal.pone.0154189. eCollection 2016. PLoS One. 2016. PMID: 27111450 Free PMC article.
-
The immunosuppressant rapamycin blocks in vitro responses to hematopoietic cytokines and inhibits recovering but not steady-state hematopoiesis in vivo.Blood. 1994 Sep 1;84(5):1543-52. Blood. 1994. PMID: 7520778
Cited by
-
Stage-specific GATA3 induction promotes ILC2 development after lineage commitment.Nat Commun. 2024 Jul 5;15(1):5610. doi: 10.1038/s41467-024-49881-y. Nat Commun. 2024. PMID: 38969652 Free PMC article.
-
Reactive myelopoiesis and FX-expressing macrophages triggered by chemotherapy promote cancer lung metastasis.JCI Insight. 2023 May 8;8(9):e167499. doi: 10.1172/jci.insight.167499. JCI Insight. 2023. PMID: 36976637 Free PMC article.
-
Reactive granulopoiesis depends on T-cell production of IL-17A and neutropenia-associated alteration of gut microbiota.Proc Natl Acad Sci U S A. 2022 Nov 29;119(48):e2211230119. doi: 10.1073/pnas.2211230119. Epub 2022 Nov 21. Proc Natl Acad Sci U S A. 2022. PMID: 36409919 Free PMC article.
-
In vivo induction of activin A-producing alveolar macrophages supports the progression of lung cell carcinoma.Nat Commun. 2023 Jan 17;14(1):143. doi: 10.1038/s41467-022-35701-8. Nat Commun. 2023. PMID: 36650150 Free PMC article.
-
The bacterial microbiota regulates normal hematopoiesis via metabolite-induced type 1 interferon signaling.Blood Adv. 2022 Mar 22;6(6):1754-1765. doi: 10.1182/bloodadvances.2021006816. Blood Adv. 2022. PMID: 35143611 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

