Medical students as adverse drug event managers, learning about side effects while improving their reporting in clinical practice
- PMID: 33666715
- PMCID: PMC8233281
- DOI: 10.1007/s00210-021-02060-y
Medical students as adverse drug event managers, learning about side effects while improving their reporting in clinical practice
Abstract
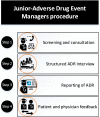
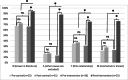
Managing adverse drug reactions (ADRs) is a challenge, especially because most healthcare professionals are insufficiently trained for this task. Since context-based clinical pharmacovigilance training has proven effective, we assessed the feasibility and effect of a creating a team of Junior-Adverse Drug Event Managers (J-ADEMs). The J-ADEM team consisted of medical students (1st-6th year) tasked with managing and reporting ADRs in hospitalized patients. Feasibility was evaluated using questionnaires. Student competence in reporting ADRs was evaluated using a case-control design and questionnaires before and after J-ADEM program participation. From Augustus 2018 to Augustus 2019, 41 students participated in a J-ADEM team and screened 136 patients and submitted 65 ADRs reports to the Netherlands Pharmacovigilance Center Lareb. Almost all patients (n = 61) found it important that "their" ADR was reported, and all (n = 62) patients felt they were taken seriously by the J-ADEM team. Although attending physicians agreed that the ADRs should have been reported, they did not do so themselves mainly because of a "lack of knowledge and attitudes" (50%) and "excuses made by healthcare professionals" (49%). J-ADEM team students were significantly more competent than control students in managing ADRs and correctly applying all steps for diagnosing ADRs (control group 38.5% vs. intervention group 83.3%, p < 0.001). The J-ADEM team is a feasible approach for detecting and managing ADRs in hospital. Patients were satisfied with the care provided, physicians were supported in their ADR reporting obligations, and students acquired relevant basic and clinical pharmacovigilance skills and knowledge, making it a win-win-win intervention.
Keywords: Clinical pharmacology; Medical education; Pharmacotherapy; Pharmacovigilance; Reporting ADRs.
Conflict of interest statement
None declared.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

