Perampanel and pregnancy
- PMID: 33666943
- PMCID: PMC7986165
- DOI: 10.1111/epi.16821
Perampanel and pregnancy
Abstract
Objective: The objective was to summarize pregnancy and fetal/postnatal outcomes following maternal perampanel exposure using preclinical and clinical data, and to use physiologically based pharmacokinetic (PBPK) modeling to improve understanding of perampanel pharmacokinetics (PK) during pregnancy.
Methods: Preclinical developmental studies with perampanel were conducted in pregnant rats and rabbits. Clinical data were collated from the Eisai global perampanel safety database, comprising reports of perampanel exposure during pregnancy from routine clinical settings, interventional studies, and non-interventional post-marketing studies, searched for events coded to Medical Dictionary for Regulatory Activities (MedDRA) high-level group terms of Pregnancy, Labor, Delivery, and Postpartum Conditions and/or the Standardized MedDRA Query terms of Congenital, Familiar, and Genetic Disorders. A PBPK model was used to predict clinical perampanel PK throughout pregnancy.
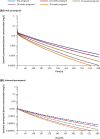
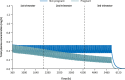
Results: Preclinical studies indicated that perampanel may be linked with post-implantation loss and/or some specific physical development delays but not fertility and early embryonic development. As of August 31, 2018, 96 pregnancies in 90 women receiving perampanel had been reported. No concomitant medications were reported in 26 (28.9%) women taking perampanel. Overall, 43 pregnancies reached full term (all normal live births), 28 did not reach term (induced abortion, n = 18; spontaneous miscarriage, n = 6; incomplete spontaneous miscarriage, n = 2; premature delivery, n = 1; stillbirth [Fallot's tetralogy], n = 1), 18 were lost to follow-up, and seven were ongoing at data cut-off. Adverse events were reported in five full-term neonates (low Apgar score, n = 2; fatal neonatal aspiration, n = 1; cystic fibrosis and congenital deafness, n = 1; poor sucking reflex and shallow breathing, n = 1). PK simulations predicted perampanel exposure decreases throughout pregnancy and is up to four- and three-fold lower towards the end of pregnancy compared with non-pregnant women for total and unbound perampanel, respectively.
Significance: These data provide preliminary information on perampanel use during pregnancy and should be interpreted with caution. Further outcome data are required to estimate the prevalence of adverse pregnancy outcomes with perampanel exposure.
Keywords: adverse events; epilepsy; perampanel; physiologically based pharmacokinetics; pregnancy.
© 2021 International League Against Epilepsy.
Conflict of interest statement
B.V. has received research support from Biogen MA Inc., Cavion LLC, Engage Therapeutics Inc., Neurelis, Ovid Therapeutics, SK Life Science Inc., UCB Biopharma SPRL, and UCB Biosciences Inc. T.T. has received speaker's honoraria to his institution from Eisai, Sandoz, Sanofi, Sun Pharmaceutical Industries Ltd, and UCB, and research support from Bial, CURE, Eisai, the European Union (ESBACE), GlaxoSmithKline, Stockholm County Council, Teva, and UCB. C.D. and E.S. are employees of Eisai Inc. T.J.O. has received research and speaker honoraria from Eisai and UCB Pharma.
Figures
References
-
- Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

