Retrograde ERK activation waves drive base-to-apex multicellular flow in murine cochlear duct morphogenesis
- PMID: 33667159
- PMCID: PMC7935486
- DOI: 10.7554/eLife.61092
Retrograde ERK activation waves drive base-to-apex multicellular flow in murine cochlear duct morphogenesis
Abstract
A notable example of spiral architecture in organs is the mammalian cochlear duct, where the morphology is critical for hearing function. Genetic studies have revealed necessary signaling molecules, but it remains unclear how cellular dynamics generate elongating, bending, and coiling of the cochlear duct. Here, we show that extracellular signal-regulated kinase (ERK) activation waves control collective cell migration during the murine cochlear duct development using deep tissue live-cell imaging, Förster resonance energy transfer (FRET)-based quantitation, and mathematical modeling. Long-term FRET imaging reveals that helical ERK activation propagates from the apex duct tip concomitant with the reverse multicellular flow on the lateral side of the developing cochlear duct, resulting in advection-based duct elongation. Moreover, model simulations, together with experiments, explain that the oscillatory wave trains of ERK activity and the cell flow are generated by mechanochemical feedback. Our findings propose a regulatory mechanism to coordinate the multicellular behaviors underlying the duct elongation during development.
Keywords: FRET imaging; MAPK/ERK; developmental biology; mathematical modeling; mechano-chemical feedbacks; morphogenesis; mouse; multicellular flow.
© 2021, Ishii et al.
Conflict of interest statement
MI, TT, MM, TH No competing interests declared
Figures

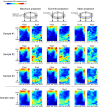
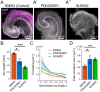
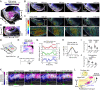


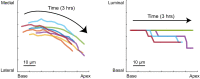
References
-
- Barrett SD, Bridges AJ, Dudley DT, Saltiel AR, Fergus JH, Flamme CM, Delaney AM, Kaufman M, LePage S, Leopold WR, Przybranowski SA, Sebolt-Leopold J, Van Becelaere K, Doherty AM, Kennedy RM, Marston D, Howard WA, Smith Y, Warmus JS, Tecle H. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorganic & Medicinal Chemistry Letters. 2008;18:6501–6504. doi: 10.1016/j.bmcl.2008.10.054. - DOI - PubMed
-
- Boocock D, Hino N, Ruzickova N, Hirashima T, Hannezo E. Theory of mechanochemical patterning and optimal migration in cell monolayers. Nature Physics. 2021;17:267–274. doi: 10.1038/s41567-020-01037-7. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

