Mitochondria as Signaling Organelles Control Mammalian Stem Cell Fate
- PMID: 33667360
- PMCID: PMC7944920
- DOI: 10.1016/j.stem.2021.02.011
Mitochondria as Signaling Organelles Control Mammalian Stem Cell Fate
Abstract
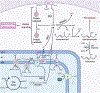
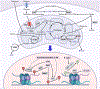
Recent evidence supports the notion that mitochondrial metabolism is necessary for the determination of stem cell fate. Historically, mitochondrial metabolism is linked to the production of ATP and tricarboxylic acid (TCA) cycle metabolites to support stem cell survival and growth, respectively. However, it is now clear that beyond these canonical roles, mitochondria as signaling organelles dictate stem cell fate and function. In this review, we focus on key conceptual ideas on how mitochondria control mammalian stem cell fate and function through reactive oxygen species (ROS) generation, TCA cycle metabolite production, NAD+/NADH ratio regulation, pyruvate metabolism, and mitochondrial dynamics.
Keywords: L-2-HG; ROS; TCA cycle; acetyl-CoA; epigenetics; mitochondrial dynamics; pyruvate.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




References
-
- Ahlqvist KJ, Hämäläinen RH, Yatsuga S, Uutela M, Terzioglu M, Götz A, Forsström S, Salven P, Angers-Loustau A, Kopra OH, et al. (2012). Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell metabolism 15, 100–109. - PubMed
-
- Avgustinova A, and Benitah SA (2016). Epigenetic control of adult stem cell function. Nature reviews. Molecular cell biology 17, 643–658. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

