An expanded universe of cancer targets
- PMID: 33667368
- PMCID: PMC8066437
- DOI: 10.1016/j.cell.2021.02.020
An expanded universe of cancer targets
Abstract
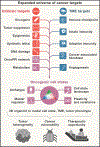
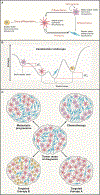
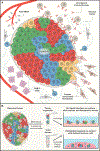
The characterization of cancer genomes has provided insight into somatically altered genes across tumors, transformed our understanding of cancer biology, and enabled tailoring of therapeutic strategies. However, the function of most cancer alleles remains mysterious, and many cancer features transcend their genomes. Consequently, tumor genomic characterization does not influence therapy for most patients. Approaches to understand the function and circuitry of cancer genes provide complementary approaches to elucidate both oncogene and non-oncogene dependencies. Emerging work indicates that the diversity of therapeutic targets engendered by non-oncogene dependencies is much larger than the list of recurrently mutated genes. Here we describe a framework for this expanded list of cancer targets, providing novel opportunities for clinical translation.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.B.M. is a science advisory board (SAB) member/consultant with AstraZeneca, Chrysallis Biotechnology, GSK, ImmunoMET, Ionis, Lilly, PDX Pharmaceuticals, Signalchem Lifesciences, Symphogen, Tarveda, Turbine, and Zentalis Pharmaceuticals; has stock/options/financial engagement with Catena Pharmaceuticals, ImmunoMet, SignalChem, and Tarveda; has licensed technology: HRD assay to Myriad Genetics and DSP patents with Nanostring; has sponsored research: Nanostring Center of Excellence and Ionis (Provision of tool compounds); and clinical trials support (funding or in kind) with AstraZeneca, Genentech, GSK, Lilly. S.L.S. is a shareholder and serves on the board of directors of Jnana Therapeutics; is a shareholder of Forma Therapeutics and Decibel Therapeutics; is a shareholder and advises Kojin Therapeutics, Kisbee Therapeutics, and Eikonizo Therapeutics; serves on the SABs of Eisai Co., Ono Pharma Foundation, Exo Therapeutics, and F-Prime Capital Partners; serves on the board of advisers of the Genomics Institute of the Novartis Research Foundation; and is a Novartis Faculty Scholar. W.C.H. is a consultant for ThermoFisher, Solasta, MPM Capital, iTeos, RAPPTA Therapeutics, Jubilant Therapeutics, and Paraxel and is a scientific founder and serves on the SAB for KSQ Therapeutics. J.S.B. is a founder and director of Neochromosome and holds an ownership equity interest in the company. A.C. is the founder and an equity holder in DarwinHealth, a company that has licensed algorithms for the analysis of regulatory networks and master regulator proteins from Columbia University. Columbia University is also an equity holder in DarwinHealth. C.J. Kemp is a founder and an equity holder in SEngine Precision Medicine, a company that harnesses 3D organoid technology and AI for more effective treatment options and accelerated drug development. W.K. and P.T. receive research support from Pfizer Oncology. W.A.W. is co-founder of StemSynergy Therapeutics. B.J.D. is an SAB member of Aileron Therapeutics, Therapy Architects (ALLCRON), Cepheid, Vivid Biosciences, Celgene, RUNX1 Research Program, Novartis, Gilead Sciences (inactive), Monojul (inactive); is an SAB member and holds stock in Aptose Biosciences, Blueprint Medicines, EnLiven Therapeutics, Iterion Therapeutics, Third Coast Therapeutics, GRAIL (SAB inactive); is a scientific founder of MolecularMD (inactive, acquired by ICON); is a member of the board of directors holds stock in Amgen; is a member of the board of directors of Burroughs Wellcome Fund and CureOne; is a member of the Joint Steering Committee of Beat AML LLS; is a founder of VB Therapeutics; has a sponsored research agreement for EnLiven Therapeutics; has clinical trial funding from Novartis, Bristol-Myers Squibb, Pfizer; and collects royalties from patent 6958335 (Novartis) and OHSU and Merck and one CytoImage exclusive license.
Figures
References
-
- Arbour KC, and Riely GJ (2019). Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA 322, 764–774. - PubMed
-
- Arendt D, Musser JM, Baker CVH, Bergman A, Cepko C, Erwin DH, Pavlicev M, Schlosser G, Widder S, Laubichler MD, et al. (2016). The origin and evolution of cell types. Nat Rev Genet 17, 744–757. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

