MDMX acts as a pervasive preleukemic-to-acute myeloid leukemia transition mechanism
- PMID: 33667384
- PMCID: PMC8575661
- DOI: 10.1016/j.ccell.2021.02.006
MDMX acts as a pervasive preleukemic-to-acute myeloid leukemia transition mechanism
Abstract
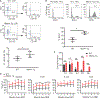
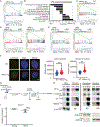
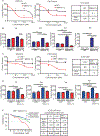
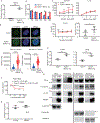
MDMX is overexpressed in the vast majority of patients with acute myeloid leukemia (AML). We report that MDMX overexpression increases preleukemic stem cell (pre-LSC) number and competitive advantage. Utilizing five newly generated murine models, we found that MDMX overexpression triggers progression of multiple chronic/asymptomatic preleukemic conditions to overt AML. Transcriptomic and proteomic studies revealed that MDMX overexpression exerts this function, unexpectedly, through activation of Wnt/β-Catenin signaling in pre-LSCs. Mechanistically, MDMX binds CK1α and leads to accumulation of β-Catenin in a p53-independent manner. Wnt/β-Catenin inhibitors reverse MDMX-induced pre-LSC properties, and synergize with MDMX-p53 inhibitors. Wnt/β-Catenin signaling correlates with MDMX expression in patients with preleukemic myelodysplastic syndromes and is associated with increased risk of progression to AML. Our work identifies MDMX overexpression as a pervasive preleukemic-to-AML transition mechanism in different genetically driven disease subtypes, and reveals Wnt/β-Catenin as a non-canonical MDMX-driven pathway with therapeutic potential for progression prevention and cancer interception.
Keywords: CK1α; MDMX; acute myeloid leukemia; cancer interception; myelodysplastic syndromes; precision prevention; preleukemia; preleukemic stem cells; targeted therapy; β-Catenin.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.A.C. is a past employee of Aileron Therapeutics. J.C. is currently an employee of Stelexis Therapeutics. ALRN-6924 was provided to U.S. from Aileron Therapeutics. U.S. has received research funding from GlaxoSmithKline, Bayer HealthCare, Aileron Therapeutics, and Novartis; has received compensation for consultancy services and for serving on scientific advisory boards from GlaxoSmithKline, Bayer Healthcare, Celgene, Aileron Therapeutics, Stelexis Therapeutics, and Pieris Pharmaceuticals; and has equity ownership in and is serving on the board of directors of Stelexis Therapeutics.
Figures




References
-
- Bacher U, Haferlach T, Schoch C, Kern W. & Schnittger S. (2006). Implications of NRAS mutations in AML: a study of 2502 patients. Blood. 107, 3847–3853. - PubMed
-
- Bereshchenko O, Mancini E, Moore S, Bilbao D, Mansson R, Luc S, Grover A, Jacobsen SE, Bryder D. & Nerlov C. (2009). Hematopoietic stem cell expansion precedes the generation of committed myeloid leukemia-initiating cells in C/EBPalpha mutant AML. Cancer Cell. 16, 390–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

