IgE blockade with omalizumab reduces pruritus related to immune checkpoint inhibitors and anti-HER2 therapies
- PMID: 33667669
- PMCID: PMC9282165
- DOI: 10.1016/j.annonc.2021.02.016
IgE blockade with omalizumab reduces pruritus related to immune checkpoint inhibitors and anti-HER2 therapies
Abstract
Background: Immunoglobulin E (IgE) blockade with omalizumab has demonstrated clinical benefit in pruritus-associated dermatoses (e.g. atopic dermatitis, bullous pemphigoid, urticaria). In oncology, pruritus-associated cutaneous adverse events (paCAEs) are frequent with immune checkpoint inhibitors (CPIs) and targeted anti-human epidermal growth factor receptor 2 (HER2) therapies. Thus, we sought to evaluate the efficacy and safety of IgE blockade with omalizumab in cancer patients with refractory paCAEs related to CPIs and anti-HER2 agents.
Patients and methods: Patients included in this multicenter retrospective analysis received monthly subcutaneous injections of omalizumab for CPI or anti-HER2 therapy-related grade 2/3 pruritus that was refractory to topical corticosteroids plus at least one additional systemic intervention. To assess clinical response to omalizumab, we used the Common Terminology Criteria for Adverse Events version 5.0. The primary endpoint was defined as reduction in the severity of paCAEs to grade 1/0.
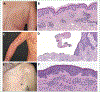
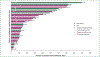
Results: A total of 34 patients (50% female, median age 67.5 years) received omalizumab for cancer therapy-related paCAEs (71% CPIs; 29% anti-HER2). All had solid tumors (29% breast, 29% genitourinary, 15% lung, 26% other), and most (n = 18, 64%) presented with an urticarial phenotype. In total 28 of 34 (82%) patients responded to omalizumab. The proportion of patients receiving oral corticosteroids as supportive treatment for management of paCAEs decreased with IgE blockade, from 50% to 9% (P < 0.001). Ten of 32 (31%) patients had interruption of oncologic therapy due to skin toxicity; four of six (67%) were successfully rechallenged following omalizumab. There were no reports of anaphylaxis or hypersensitivity reactions related to omalizumab.
Conclusions: IgE blockade with omalizumab demonstrated clinical efficacy and was well tolerated in cancer patients with pruritus related to CPIs and anti-HER2 therapies.
Keywords: IgE; bullous pemphigoid; cutaneous adverse event; eczema; pruritus; urticaria.
Copyright © 2021 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure AM has an advisory board role in AstraZeneca, receives research funding from Incyte, and is supported by a Dermatology Foundation Career Development Award. EQ receives royalties from UpToDate. SAF receives research funding from AstraZeneca and Genentech/Roche, has a consulting/advisory relationship with Merck, and has ownership interests in Urogen, Allogene Therapeutics, Neogene Therapeutics, Kronos Bio, InconOVir and Vida Ventures. MHV reports receiving commercial research support from Pfizer and honoraria from Pfizer, Exelixis, Eisai, Calithera Biosciences, and Corvus Pharmaceuticals. AD discloses honoraria/advisory board participation for Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millennium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, and MORE Health; associated research funding paid to the institution from Pfizer, Exelixis, GlaxoSmithKline, Teva, Taiho, and PharmaMar; research for Foundation Medicine; royalties from Wolters Kluwer; expenses from Merck and Puma; and CME honoraria from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, and Research to Practice. MDH receives research funding from Bristol Myers Squibb; is a paid consultant to Merck, Bristol Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax, Mirati, Blueprint, Achilles Therapeutics, PACT Pharma, Immunai, and Shattuck Labs; receives travel support/honoraria from AstraZeneca, Eli Lilly, Merck, and BMS; and a patent has been filed by MSK related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx. EAC reports consultancy fees from Pfizer, Novartis, Bristol Myers Squibb, COTA, Genentech-Roche, and Heron Therapeutics. DYML has been a consultant for Genentech and Novartis. MEL has a consultant role with Johnson and Johnson, Novocure, QED, Bicara, Janssen, Novartis, F. Hoffmann-La Roche AG, EMD Serono, Astrazeneca, Innovaderm, Deciphera, DFB, Azitra, Kintara, RBC/La Roche Posay, Trifecta, Varsona, Genentech, Loxo, Seattle Genetics, Lutris, OnQuality, Azitra, Roche, NCODA, Apricity, Oncoderm Labs, Hoth Therapeutics. Dr. Lacouture also receives research funding from Lutris, Paxman, Novocure, J&J, US Biotest, OQL, Novartis and AZ. All other authors have declared no conflicts of interest.
Figures

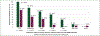
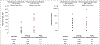
Comment in
-
IgE blockade in the management of eosinophil-associated recalcitrant pruritus due to medical tumor therapy.Ann Oncol. 2021 Jun;32(6):696-697. doi: 10.1016/j.annonc.2021.04.007. Epub 2021 Apr 18. Ann Oncol. 2021. PMID: 33882330 No abstract available.
Similar articles
-
Rapid Disease Control in First-Line Therapy-Resistant Mucous Membrane Pemphigoid and Bullous Pemphigoid with Omalizumab as Add-On Therapy: A Case Series Of 13 Patients.Front Immunol. 2022 Apr 20;13:874108. doi: 10.3389/fimmu.2022.874108. eCollection 2022. Front Immunol. 2022. PMID: 35514989 Free PMC article.
-
Checkpoint inhibitor-associated bullous cutaneous immune-related adverse events: a multicentre observational study.Br J Dermatol. 2022 Dec;187(6):981-987. doi: 10.1111/bjd.21836. Epub 2022 Sep 6. Br J Dermatol. 2022. PMID: 35976170
-
Dermatologic uses of omalizumabtitle.J Dermatolog Treat. 2017 Jun;28(4):332-337. doi: 10.1080/09546634.2016.1249819. Epub 2016 Nov 7. J Dermatolog Treat. 2017. PMID: 27759482 Review.
-
The efficacy of omalizumab in Cutaneous Mastocytosis: A case series.Dermatol Ther. 2019 May;32(3):e12848. doi: 10.1111/dth.12848. Epub 2019 Feb 20. Dermatol Ther. 2019. PMID: 30697883
-
IgE-Related Chronic Diseases and Anti-IgE-Based Treatments.J Immunol Res. 2016;2016:8163803. doi: 10.1155/2016/8163803. Epub 2016 Dec 21. J Immunol Res. 2016. PMID: 28097159 Free PMC article. Review.
Cited by
-
Safety of Immunomodulatory Systemic Therapies Used in the Management of Immune-Related Cutaneous Adverse Events.Pharmaceuticals (Basel). 2023 Nov 15;16(11):1610. doi: 10.3390/ph16111610. Pharmaceuticals (Basel). 2023. PMID: 38004475 Free PMC article. Review.
-
Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article.Curr Oncol. 2022 Apr 18;29(4):2871-2886. doi: 10.3390/curroncol29040234. Curr Oncol. 2022. PMID: 35448208 Free PMC article. Review.
-
Case report: Bullous pemphigoid associated with sintilimab therapy for pMMR/MSS colorectal cancer.Front Oncol. 2023 Mar 14;13:1124730. doi: 10.3389/fonc.2023.1124730. eCollection 2023. Front Oncol. 2023. PMID: 36998454 Free PMC article.
-
Severe cutaneous adverse reactions.Nat Rev Dis Primers. 2024 Apr 25;10(1):30. doi: 10.1038/s41572-024-00514-0. Nat Rev Dis Primers. 2024. PMID: 38664435 Review.
-
Challenging Dermatologic Considerations Associated with Immune Checkpoint Inhibitors.Am J Clin Dermatol. 2022 Sep;23(5):707-717. doi: 10.1007/s40257-022-00706-y. Epub 2022 Jun 16. Am J Clin Dermatol. 2022. PMID: 35708849 Review.
References
-
- Andre F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019;380(20):1929–1940. - PubMed
-
- Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol 2014;71(1): 161–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

