Clinical significance of measuring serum cytokine levels as inflammatory biomarkers in adult and pediatric COVID-19 cases: A review
- PMID: 33667962
- PMCID: PMC7901304
- DOI: 10.1016/j.cyto.2021.155478
Clinical significance of measuring serum cytokine levels as inflammatory biomarkers in adult and pediatric COVID-19 cases: A review
Abstract
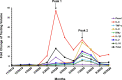
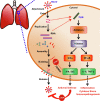
Coronavirus disease 2019 (COVID-19) is a rapidly evolving infectious/inflammatory disorder which has turned into a global pandemic. With severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as its etiologic agent, severe COVID-19 cases usually develop uncontrolled inflammatory responses and cytokine storm-like syndromes. Measuring serum levels of pro-inflammatory cytokines (e.g., IL-6 and others) as inflammatory biomarkers may have several potential applications in the management of COVID-19, including risk assessment, monitoring of disease progression, determination of prognosis, selection of therapy and prediction of response to treatment.This is especially true for pediatric patients with COVID-19 associated Kawasaki-like disease and similar syndromes. In this report, we review the current knowledge of COVID-19 associated cytokines, their roles in host immune and inflammatory responses, the clinical significance and utility of cytokine immunoassays in adult and pediatric COVID-19 patients, as well as the challenges and pitfalls in implementation and interpretation of cytokine immunoassays. Given that cytokines are implicated in different immunological disorders and diseases, it is challenging to interpret the multiplex cytokine data for COVID-19 patients. Also, it should be taken into consideration that biological and technical variables may affect the commutability of cytokine immunoassays and enhance complexity of cytokine immunoassay interpretation. It is recommended that the same method, platform and laboratory should be used when monitoring differences in cytokine levels between groups of individuals or for the same individual over time. It may be important to correlate cytokine profiling data with the SARS-CoV-2 nucleic acid amplification testing and imaging observations to make an accurate interpretation of the inflammatory status and disease progression in COVID-19 patients.
Keywords: COVID-19; Cytokine; Inflammatory biomarkers; Kawasaki disease; Multiplex cytokine analysis.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Martins T.B., Anderson J.L., Muhlestein J.B., Horne B.D., Carlquist J.F., Roberts W.L., Hill H.R. Risk factor analysis of plasma cytokines in patients with coronary artery disease by a multiplexed fluorescent immunoassay. Am. J. Clin. Pathol. 2006;125(6):906–913. doi: 10.1309/Q3E6-KF0Q-D3U3-YL6T. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

