Direct Regulation of DNA Repair by E2F and RB in Mammals and Plants: Core Function or Convergent Evolution?
- PMID: 33668093
- PMCID: PMC7956360
- DOI: 10.3390/cancers13050934
Direct Regulation of DNA Repair by E2F and RB in Mammals and Plants: Core Function or Convergent Evolution?
Abstract
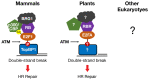
Members of the E2F transcription factor family regulate the expression of genes important for DNA replication and mitotic cell division in most eukaryotes. Homologs of the retinoblastoma (RB) tumor suppressor inhibit the activity of E2F factors, thus controlling cell cycle progression. Organisms such as budding and fission yeast have lost genes encoding E2F and RB, but have gained genes encoding other proteins that take on E2F and RB cell cycle-related functions. In addition to regulating cell proliferation, E2F and RB homologs have non-canonical functions outside the mitotic cell cycle in a variety of eukaryotes. For example, in both mammals and plants, E2F and RB homologs localize to DNA double-strand breaks (DSBs) and directly promote repair by homologous recombination (HR). Here, we discuss the parallels between mammalian E2F1 and RB and their Arabidopsis homologs, E2FA and RB-related (RBR), with respect to their recruitment to sites of DNA damage and how they help recruit repair factors important for DNA end resection. We also explore the question of whether this role in DNA repair is a conserved ancient function of the E2F and RB homologs in the last eukaryotic common ancestor or whether this function evolved independently in mammals and plants.
Keywords: ATM; BRG1; E2F1; TopBP1; homologous recombination; p300/CBP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

