Unexpected Benefits of Multiport Synchrotron Microbeam Radiation Therapy for Brain Tumors
- PMID: 33668110
- PMCID: PMC7956531
- DOI: 10.3390/cancers13050936
Unexpected Benefits of Multiport Synchrotron Microbeam Radiation Therapy for Brain Tumors
Abstract
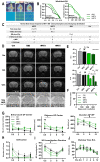
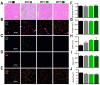
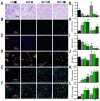
Delivery of high-radiation doses to brain tumors via multiple arrays of synchrotron X-ray microbeams permits huge therapeutic advantages. Brain tumor (9LGS)-bearing and normal rats were irradiated using a conventional, homogeneous Broad Beam (BB), or Microbeam Radiation Therapy (MRT), then studied by behavioral tests, MRI, and histopathology. A valley dose of 10 Gy deposited between microbeams, delivered by a single port, improved tumor control and median survival time of tumor-bearing rats better than a BB isodose. An increased number of ports and an accumulated valley dose maintained at 10 Gy delayed tumor growth and improved survival. Histopathologically, cell death, vascular damage, and inflammatory response increased in tumors. At identical valley isodose, each additional MRT port extended survival, resulting in an exponential correlation between port numbers and animal lifespan (r2 = 0.9928). A 10 Gy valley dose, in MRT mode, delivered through 5 ports, achieved the same survival as a 25 Gy BB irradiation because of tumor dose hot spots created by intersecting microbeams. Conversely, normal tissue damage remained minimal in all the single converging extratumoral arrays. Multiport MRT reached exceptional ~2.5-fold biological equivalent tumor doses. The unique normal tissue sparing and therapeutic index are eminent prerequisites for clinical translation.
Keywords: brain tumor control; dose equivalence; normal tissue sparing; synchrotron microbeam radiation therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ostrom Q.T., Cioffi G., Gittleman H., Patil N., Waite K., Kruchko C., Barnholtz-sloan J.S., Comprehensive C., Western C. Neuro-Oncology CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21:v1–v100. doi: 10.1093/neuonc/noz150. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

