Alveolar Epithelial Denudation Is a Major Factor in the Pathogenesis of Pleuroparenchymal Fibroelastosis
- PMID: 33668178
- PMCID: PMC7956653
- DOI: 10.3390/jcm10050895
Alveolar Epithelial Denudation Is a Major Factor in the Pathogenesis of Pleuroparenchymal Fibroelastosis
Abstract
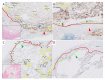
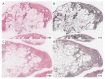
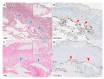
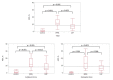
The pathogenesis of pleuroparenchymal fibroelastosis (PPFE), a rare interstitial lung disease, remains unclear. Based on previous reports and our experience, we hypothesized that alveolar epithelial denudation (AED) was involved in the pathogenesis of PPFE. This multicenter retrospective study investigated the percentage of AED and the features of the denudated areas in 26 PPFE cases, 30 idiopathic pulmonary fibrosis (IPF) cases, and 29 controls. PPFE patients had lower forced vital capacities and higher residual volume/total lung capacities in pulmonary function tests compared to IPF and control patients. Histopathologically, subpleural fibroelastosis was observed in PPFE, and AED was observed in 12.01% of cases in the subpleural or interlobular septa regardless of fibroelastosis. The percentage of AED in the PPFE group was significantly higher than that in the IPF group (6.84%; p = 0.03) and the normal group (1.19%; p < 0.001). In the IPF group, the percentage of AED and the presence of PPFE-like lesions in the upper lobes were examined radiologically, but no correlation was found. We showed that AED frequently occurred in PPFE. AED was less frequent in IPF, which, in combination with imaging data, suggests that PPFE may have a different pathogenesis from IPF.
Keywords: classification; epithelial denudation; epithelial detachment; idiopathic pulmonary fibrosis; image analysis; pathogenic mechanism; pathology; pleuroparenchymal fibroelastosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The similarities and differences between pleuroparenchymal fibroelastosis and idiopathic pulmonary fibrosis.Chron Respir Dis. 2019 Jan-Dec;16:1479973119867945. doi: 10.1177/1479973119867945. Chron Respir Dis. 2019. PMID: 31387379 Free PMC article. Review.
-
Clinical significance of radiological pleuroparenchymal fibroelastosis pattern in interstitial lung disease patients registered for lung transplantation: a retrospective cohort study.Respir Res. 2018 Aug 30;19(1):162. doi: 10.1186/s12931-018-0860-6. Respir Res. 2018. PMID: 30165854 Free PMC article.
-
Clinical, radiological and pathological features of idiopathic and secondary interstitial pneumonia with pleuroparenchymal fibroelastosis in patients undergoing lung transplantation.Histopathology. 2022 Mar;80(4):665-676. doi: 10.1111/his.14595. Epub 2022 Jan 5. Histopathology. 2022. PMID: 34747513
-
Pleuroparenchymal fibroelastosis-like lesions in patients with interstitial pneumonia diagnosed by multidisciplinary discussion with surgical lung biopsy.Eur J Radiol Open. 2020 Dec 11;7:100298. doi: 10.1016/j.ejro.2020.100298. eCollection 2020. Eur J Radiol Open. 2020. PMID: 33354595 Free PMC article.
-
Pleuroparenchymal Fibroelastosis of the Lung: A Review.Arch Pathol Lab Med. 2016 Aug;140(8):849-53. doi: 10.5858/arpa.2015-0166-RS. Arch Pathol Lab Med. 2016. PMID: 27472241 Review.
Cited by
-
The Role of Epithelial Damage in the Pulmonary Immune Response.Cells. 2021 Oct 15;10(10):2763. doi: 10.3390/cells10102763. Cells. 2021. PMID: 34685744 Free PMC article. Review.
-
Machine-Learning-Based Classification Model to Address Diagnostic Challenges in Transbronchial Lung Biopsy.Cancers (Basel). 2024 Feb 9;16(4):731. doi: 10.3390/cancers16040731. Cancers (Basel). 2024. PMID: 38398122 Free PMC article.
References
-
- Amitani R., Niimi A., Kuse F. Idiopathic pulmonary upper lobe fibrosis (IPUF) Kokyu. 1992;11:693–699.
-
- Travis W.D., Costabel U., Hansell D.M., King T.E., Jr., Lynch D.A., Nicholson A.G., Valeyre D. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

