The Many Facets of Therapy Resistance and Tumor Recurrence in Glioblastoma
- PMID: 33668200
- PMCID: PMC7995978
- DOI: 10.3390/cells10030484
The Many Facets of Therapy Resistance and Tumor Recurrence in Glioblastoma
Abstract
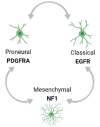
Glioblastoma (GBM) is the most lethal type of primary brain cancer. Standard care using chemo- and radio-therapy modestly increases the overall survival of patients; however, recurrence is inevitable, due to treatment resistance and lack of response to targeted therapies. GBM therapy resistance has been attributed to several extrinsic and intrinsic factors which affect the dynamics of tumor evolution and physiology thus creating clinical challenges. Tumor-intrinsic factors such as tumor heterogeneity, hypermutation, altered metabolomics and oncologically activated alternative splicing pathways change the tumor landscape to facilitate therapy failure and tumor progression. Moreover, tumor-extrinsic factors such as hypoxia and an immune-suppressive tumor microenvironment (TME) are the chief causes of immunotherapy failure in GBM. Amid the success of immunotherapy in other cancers, GBM has occurred as a model of resistance, thus focusing current efforts on not only alleviating the immunotolerance but also evading the escape mechanisms of tumor cells to therapy, caused by inter- and intra-tumoral heterogeneity. Here we review the various mechanisms of therapy resistance in GBM, caused by the continuously evolving tumor dynamics as well as the complex TME, which cumulatively contribute to GBM malignancy and therapy failure; in an attempt to understand and identify effective therapies for recurrent GBM.
Keywords: Glioblastoma; hypermutation; hypoxia; metabolism; recurrence; resistance; splicing; tumor heterogeneity; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
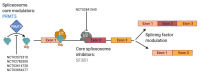

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

