Advances in Sensing, Response and Regulation Mechanism of Salt Tolerance in Rice
- PMID: 33668247
- PMCID: PMC7956267
- DOI: 10.3390/ijms22052254
Advances in Sensing, Response and Regulation Mechanism of Salt Tolerance in Rice
Abstract
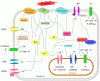
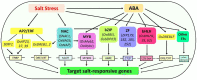
Soil salinity is a serious menace in rice production threatening global food security. Rice responses to salt stress involve a series of biological processes, including antioxidation, osmoregulation or osmoprotection, and ion homeostasis, which are regulated by different genes. Understanding these adaptive mechanisms and the key genes involved are crucial in developing highly salt-tolerant cultivars. In this review, we discuss the molecular mechanisms of salt tolerance in rice-from sensing to transcriptional regulation of key genes-based on the current knowledge. Furthermore, we highlight the functionally validated salt-responsive genes in rice.
Keywords: antioxidation; ion homeostasis; osmoregulation; rice; salinity; sensing; signaling; transcription factors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Transcriptome analysis in different rice cultivars provides novel insights into desiccation and salinity stress responses.Sci Rep. 2016 Mar 31;6:23719. doi: 10.1038/srep23719. Sci Rep. 2016. PMID: 27029818 Free PMC article.
-
OsC2DP, a Novel C2 Domain-Containing Protein Is Required for Salt Tolerance in Rice.Plant Cell Physiol. 2019 Oct 1;60(10):2220-2230. doi: 10.1093/pcp/pcz115. Plant Cell Physiol. 2019. PMID: 31198970
-
Physiology and proteome responses of two contrasting rice mutants and their wild type parent under salt stress conditions at the vegetative stage.J Plant Physiol. 2014 Jan 1;171(1):31-44. doi: 10.1016/j.jplph.2013.07.014. Epub 2013 Oct 3. J Plant Physiol. 2014. PMID: 24094368
-
Physiological and Molecular Mechanisms of Rice Tolerance to Salt and Drought Stress: Advances and Future Directions.Int J Mol Sci. 2024 Aug 29;25(17):9404. doi: 10.3390/ijms25179404. Int J Mol Sci. 2024. PMID: 39273349 Free PMC article. Review.
-
Advances and Challenges in the Breeding of Salt-Tolerant Rice.Int J Mol Sci. 2020 Nov 9;21(21):8385. doi: 10.3390/ijms21218385. Int J Mol Sci. 2020. PMID: 33182265 Free PMC article. Review.
Cited by
-
Exogenous Hemin alleviates NaCl stress by promoting photosynthesis and carbon metabolism in rice seedlings.Sci Rep. 2023 Mar 1;13(1):3497. doi: 10.1038/s41598-023-30619-7. Sci Rep. 2023. PMID: 36859499 Free PMC article.
-
Integrated Analysis of Transcriptome and Metabolome Reveals Molecular Mechanisms of Rice with Different Salinity Tolerances.Plants (Basel). 2023 Sep 22;12(19):3359. doi: 10.3390/plants12193359. Plants (Basel). 2023. PMID: 37836098 Free PMC article.
-
Nanoparticles as a Tool for Alleviating Plant Stress: Mechanisms, Implications, and Challenges.Plants (Basel). 2024 May 31;13(11):1528. doi: 10.3390/plants13111528. Plants (Basel). 2024. PMID: 38891334 Free PMC article. Review.
-
A salt-tolerant growth-promoting phyllosphere microbial combination from mangrove plants and its mechanism for promoting salt tolerance in rice.Microbiome. 2024 Dec 20;12(1):270. doi: 10.1186/s40168-024-01969-9. Microbiome. 2024. PMID: 39707568 Free PMC article.
-
OsSTS, a Novel Allele of Mitogen-Activated Protein Kinase Kinase 4 (OsMKK4), Controls Grain Size and Salt Tolerance in Rice.Rice (N Y). 2023 Oct 24;16(1):47. doi: 10.1186/s12284-023-00663-y. Rice (N Y). 2023. PMID: 37874376 Free PMC article.
References
-
- Zeng L., Shannon M.C., Grieve C.M. Evaluation of salt tolerance in rice genotypes by multiple agronomic parameters. Euphytica. 2002;127:235–245. doi: 10.1023/A:1020262932277. - DOI
-
- Lutts S., Kinet J.M., Bouharmont J. Effects of salt stress on growth mineral nutrition and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) cultivars differing in salinity resistance. Plant Growth Regul. 1996;19:207–218. doi: 10.1007/BF00037793. - DOI
-
- Cui H., Takeoka Y., Wada T. Effect of sodium chloride on the panicle and spikelet morphogenesis in rice. Jpn. J. Crop. Sci. 1995;64:593–600. doi: 10.1626/jcs.64.593. - DOI
-
- Khatun S., Flowers T.J. Effects of salinity on seed set in rice. Plant Cell. Environ. 1995;18:61–67. doi: 10.1111/j.1365-3040.1995.tb00544.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

