Development of a Microfluidic Platform for Trace Lipid Analysis
- PMID: 33668377
- PMCID: PMC7996208
- DOI: 10.3390/metabo11030130
Development of a Microfluidic Platform for Trace Lipid Analysis
Abstract
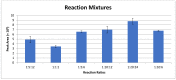
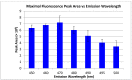
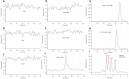
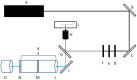
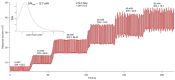
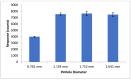
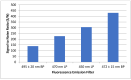
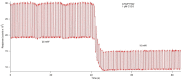
The inherent trace quantity of primary fatty acid amides found in biological systems presents challenges for analytical analysis and quantitation, requiring a highly sensitive detection system. The use of microfluidics provides a green sample preparation and analysis technique through small-volume fluidic flow through micron-sized channels embedded in a polydimethylsiloxane (PDMS) device. Microfluidics provides the potential of having a micro total analysis system where chromatographic separation, fluorescent tagging reactions, and detection are accomplished with no added sample handling. This study describes the development and the optimization of a microfluidic-laser induced fluorescence (LIF) analysis and detection system that can be used for the detection of ultra-trace levels of fluorescently tagged primary fatty acid amines. A PDMS microfluidic device was designed and fabricated to incorporate droplet-based flow. Droplet microfluidics have enabled on-chip fluorescent tagging reactions to be performed quickly and efficiently, with no additional sample handling. An optimized LIF optical detection system provided fluorescently tagged primary fatty acid amine detection at sub-fmol levels (436 amol). The use of this LIF detection provides unparalleled sensitivity, with detection limits several orders of magnitude lower than currently employed LC-MS techniques, and might be easily adapted for use as a complementary quantification platform for parallel MS-based omics studies.
Keywords: bioactive lipids; laser induced fluorescence; microfluidics; primary fatty acid amides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A miniaturized, low-cost lens tube based laser-induced fluorescence detection system for automated microfluidic analysis of primary amines.Talanta. 2022 May 1;241:123227. doi: 10.1016/j.talanta.2022.123227. Epub 2022 Jan 13. Talanta. 2022. PMID: 35101836
-
Droplet-based μChopper device with a 3D-printed pneumatic valving layer and a simple photometer for absorbance based fructosamine quantification in human serum.Analyst. 2023 Sep 25;148(19):4810-4819. doi: 10.1039/d3an01149f. Analyst. 2023. PMID: 37605899 Free PMC article.
-
Small volume low mechanical stress cytometry using computer-controlled Braille display microfluidics.Lab Chip. 2007 Nov;7(11):1497-503. doi: 10.1039/b708187a. Epub 2007 Aug 15. Lab Chip. 2007. PMID: 17960277
-
Analytical detection techniques for droplet microfluidics--a review.Anal Chim Acta. 2013 Jul 17;787:24-35. doi: 10.1016/j.aca.2013.04.064. Epub 2013 May 14. Anal Chim Acta. 2013. PMID: 23830418 Review.
-
Droplet microfluidics based microseparation systems.J Sep Sci. 2012 Jun;35(10-11):1284-93. doi: 10.1002/jssc.201200115. J Sep Sci. 2012. PMID: 22733508 Review.
Cited by
-
The Intersection of Metabolomics and Data Science.Metabolites. 2023 Aug 4;13(8):915. doi: 10.3390/metabo13080915. Metabolites. 2023. PMID: 37623859 Free PMC article.
-
Structural studies of the human α1 glycine receptor via site-specific chemical cross-linking coupled with mass spectrometry.Biophys Rep (N Y). 2024 Dec 11;4(4):100184. doi: 10.1016/j.bpr.2024.100184. Epub 2024 Oct 10. Biophys Rep (N Y). 2024. PMID: 39393591 Free PMC article.
-
Changes in lipid abundance are associated with disease progression and treatment response in chronic Trypanosoma cruzi infection.Parasit Vectors. 2024 Nov 9;17(1):459. doi: 10.1186/s13071-024-06548-3. Parasit Vectors. 2024. PMID: 39521974 Free PMC article.
References
-
- Cravatt B.F., Lerner R.A., Boger D.L. Structure Determination of an Endogenous Sleep-Inducing Lipid, cis-9-Octadecanamide (Oleamide): A Synthetic Approach to the Chemical Analysis of Trace Quantities of a Natural Product. J. Am. Chem. Soc. 1996;118:580–590. doi: 10.1021/ja9532345. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

