Cystic Fibrosis Newborn Screening in Austria Using PAP and the Numeric Product of PAP and IRT Concentrations as Second-Tier Parameters
- PMID: 33668470
- PMCID: PMC7918494
- DOI: 10.3390/diagnostics11020299
Cystic Fibrosis Newborn Screening in Austria Using PAP and the Numeric Product of PAP and IRT Concentrations as Second-Tier Parameters
Abstract
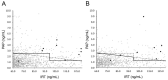
In Austria, newborns have been screened for cystic fibrosis (CF) by analyzing immunoreactive trypsinogen (IRT) from dried blood spots (DBS)s for nearly 20 years. Recently, pancreatitis-associated protein (PAP) analysis was introduced as a second-tier test with the aim of reducing recalls for second DBS cards while keeping sensitivity high. For 28 months, when IRT was elevated (65-130 ng/mL), PAP was measured from the first DBS (n = 198,927) with a two-step cut-off applied. For the last 12 months of the observation period (n = 85,421), an additional IRT×PAP cut-off was introduced. If PAP or IRT×PAP were above cut-off, a second card was analyzed for IRT and in case of elevated values identified as screen-positive. Above 130 ng/mL IRT in the first DBS, newborns were classified as screen-positive. IRT analysis of first DBS resulted in 1961 (1%) tests for PAP. In the first 16 months, 26 of 93 screen-positive were confirmed to have CF. Two false-negatives have been reported (sensitivity = 92.8%). Importantly, less than 30% of families compared to the previous IRT-IRT screening scheme had to be contacted causing distress. Adding IRT×PAP caused a marginally increased number of second cards and sweat tests to be requested during this period (15 and 3, respectively) compared to the initial IRT-PAP scheme. One case of confirmed CF was found due to IRT×PAP, demonstrating an increase in sensitivity. Thus, the relatively simple and economical algorithm presented here performs effectively and may be a useful model for inclusion of CF into NBS panels or modification of existing schemes.
Keywords: IRT-PAP; IRT×PAP; false-positives; neonatal screening; recalls.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

