α-Ketoheterocycles Able to Inhibit the Generation of Prostaglandin E2 (PGE2) in Rat Mesangial Cells
- PMID: 33668480
- PMCID: PMC7918003
- DOI: 10.3390/biom11020275
α-Ketoheterocycles Able to Inhibit the Generation of Prostaglandin E2 (PGE2) in Rat Mesangial Cells
Abstract
Prostaglandin E2 (PGE2) is a key mediator of inflammation, and consequently huge efforts have been devoted to the development of novel agents able to regulate its formation. In this work, we present the synthesis of various α-ketoheterocycles and a study of their ability to inhibit the formation of PGE2 at a cellular level. A series of α-ketobenzothiazoles, α-ketobenzoxazoles, α-ketobenzimidazoles, and α-keto-1,2,4-oxadiazoles were synthesized and chemically characterized. Evaluation of their ability to suppress the generation of PGE2 in interleukin-1β plus forskolin-stimulated mesangial cells led to the identification of one α-ketobenzothiazole (GK181) and one α-ketobenzoxazole (GK491), which are able to suppress the PGE2 generation at a nanomolar level.
Keywords: anti-inflammatory; inhibition; mesangial cells; prostaglandin E2; α-ketobenzothiazoles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



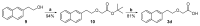
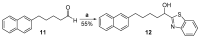

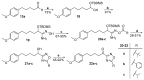


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

