Protective Role of Sphingomyelin in Eye Lens Cell Membrane Model against Oxidative Stress
- PMID: 33668553
- PMCID: PMC7918908
- DOI: 10.3390/biom11020276
Protective Role of Sphingomyelin in Eye Lens Cell Membrane Model against Oxidative Stress
Abstract
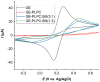
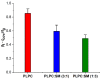
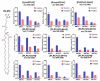
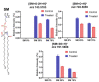
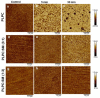
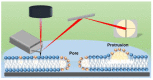
In the eye lens cell membrane, the lipid composition changes during the aging process: the proportion of sphingomyelins (SM) increases, that of phosphatidylcholines decreases. To investigate the protective role of the SMs in the lens cell membrane against oxidative damage, analytical techniques such as electrochemistry, high-resolution mass spectrometry (HR-MS), and atomic force microscopy (AFM) were applied. Supported lipid bilayers (SLB) were prepared to mimic the lens cell membrane with different fractions of PLPC/SM (PLPC: 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine). The SLBs were treated with cold physical plasma. A protective effect of 30% and 44% in the presence of 25%, and 75% SM in the bilayer was observed, respectively. PLPC and SM oxidation products were determined via HR-MS for SLBs after plasma treatment. The yield of fragments gradually decreased as the SM ratio increased. Topographic images obtained by AFM of PLPC-bilayers showed SLB degradation and pore formation after plasma treatment, no degradation was observed in PLPC/SM bilayers. The results of all techniques confirm the protective role of SM in the membrane against oxidative damage and support the idea that the SM content in lens cell membrane is increased during aging in the absence of effective antioxidant systems to protect the eye from oxidative damage and to prolong lens transparency.
Keywords: aging; atomic force microscopy; cold physical plasma; electrochemistry; eye lens cell membrane; mass spectrometry; oxidized lipids; sphingomyelin.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Babizhayev M.A., Yegorov Y.E. Reactive oxygen species and the aging eye: Specific role of metabolically active mitochondria in maintaining lens function and in the initiation of the oxidation-induced maturity onset cataract—A novel platform of mitochondria-targeted antioxidants with broad therapeutic potential for redox regulation and detoxification of oxidants in eye diseases. Am. J. Ther. 2016;23:e98–e117. doi: 10.1097/MJT.0b013e3181ea31ff. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

