Distribution, Ecology, Chemistry and Toxicology of Plant Stinging Hairs
- PMID: 33668609
- PMCID: PMC7918447
- DOI: 10.3390/toxins13020141
Distribution, Ecology, Chemistry and Toxicology of Plant Stinging Hairs
Abstract
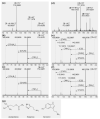
Plant stinging hairs have fascinated humans for time immemorial. True stinging hairs are highly specialized plant structures that are able to inject a physiologically active liquid into the skin and can be differentiated from irritant hairs (causing mechanical damage only). Stinging hairs can be classified into two basic types: Urtica-type stinging hairs with the classical "hypodermic syringe" mechanism expelling only liquid, and Tragia-type stinging hairs expelling a liquid together with a sharp crystal. In total, there are some 650 plant species with stinging hairs across five remotely related plant families (i.e., belonging to different plant orders). The family Urticaceae (order Rosales) includes a total of ca. 150 stinging representatives, amongst them the well-known stinging nettles (genus Urtica). There are also some 200 stinging species in Loasaceae (order Cornales), ca. 250 stinging species in Euphorbiaceae (order Malphigiales), a handful of species in Namaceae (order Boraginales), and one in Caricaceae (order Brassicales). Stinging hairs are commonly found on most aerial parts of the plants, especially the stem and leaves, but sometimes also on flowers and fruits. The ecological role of stinging hairs in plants seems to be essentially defense against mammalian herbivores, while they appear to be essentially inefficient against invertebrate pests. Stinging plants are therefore frequent pasture weeds across different taxa and geographical zones. Stinging hairs are usually combined with additional chemical and/or mechanical defenses in plants and are not a standalone mechanism. The physiological effects of stinging hairs on humans vary widely between stinging plants and range from a slight itch, skin rash (urticaria), and oedema to sharp pain and even serious neurological disorders such as neuropathy. Numerous studies have attempted to elucidate the chemical basis of the physiological effects. Since the middle of the 20th century, neurotransmitters (acetylcholine, histamine, serotonin) have been repeatedly detected in stinging hairs of Urticaceae, but recent analyses of Loasaceae stinging hair fluids revealed high variability in their composition and content of neurotransmitters. These substances can explain some of the physiological effects of stinging hairs, but fail to completely explain neuropathic effects, pointing to some yet unidentified neurotoxin. Inorganic ions (e.g., potassium) are detected in stinging hairs and could have synergistic effects. Very recently, ultrastable miniproteins dubbed "gympietides" have been reported from two species of Dendrocnide, arguably the most violently stinging plant. Gympietides are shown to be highly neurotoxic, providing a convincing explanation for Dendrocnide toxicity. For the roughly 648 remaining stinging plant species, similarly convincing data on toxicity are still lacking.
Keywords: Dendrocnide; Loasaceae; Tragia; Urtica; acetylcholine; defense mechanisms; herbivores; histamine; neurotransmitters; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Thurston E.L., Lersten N.R. The morphology and toxicology of plant stinging hairs. Bot. Rev. 1969;35:393–412. doi: 10.1007/BF02858878. - DOI
-
- Hooke R. John Martyn “Printers to the Royal Society”. Dover Pub. Co.; New York, NY, USA: 1965. Micrographia: Or Some Physiological Descriptions of Minute Bodies Made by Magnifying Glasses with Observations and Inquiries thereupon.
-
- Gorup-Besanetz E.F. Notiz über das Vorkommen der Ameisensäure in den Brennnesseln. J. Prakt. Chem. 1849;48:191–192. doi: 10.1002/prac.18490480124. - DOI
-
- Rauter J. Zur Entwicklungsgeschichte einiger Trichomgebilde. Denkschr. Akad. Wiss. 1872;31:2–49.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

